Lighting a Bunsen Burner - Experimental Skill and Investigation
advertisement

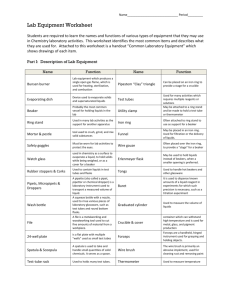
Shannon DeSmet Tammy Dudenhoffer Using a Bunsen Burner Introduction A Bunsen burner is one of the most widely used pieces of equipment in any chemistry laboratory. It is unique because it produces a hot, steady, and smokeless flame. The Bunsen burner is most often used to heat materials, but can also be used in sterilization and combustion. The focus of the following activity is to introduce you to the Bunsen burner so you can safely and successfully use it in upcoming experiments in this class. In order to use it safely and appropriately, it is important to know the correct steps on how to set it up and operate it. Safety Precautions and Inspection: Warning: Bunsen burners are involved in the most common forms of laboratory accidents. You will be working with a very hot flame that is burning Methane gas. A Bunsen burner is made out of metal, and gets extremely hot after being lit for a short period of time. To ensure you are being safe follow the guidelines below. PLACE the Bunsen burner away from any overhead shelving, equipment or light fixtures. REMOVE all papers, notebooks, combustible materials and excess chemicals from the area. TIE-BACK any long hair, dangling jewelry, or loose clothing. INSPECT hose for cracks, holes, pinched points, or any other defect and ensure that the hose fits securely on the gas valve and the Bunsen burner NOTIFY others in the laboratory that the burner will be in use. UTILIZE a sparker / lighter with extended nozzle to ignite the Bunsen burner. Never use a match to ignite burner. ADJUST the flame by turning the collar to regulate air flow and produce an appropriate flame for the experiment (typically a medium blue flame). DO NOT leave open flames unattended and never leave laboratory while burner is on. SHUT-OFF gas when its use is complete. Shannon DeSmet Tammy Dudenhoffer ALLOW the burner to cool before handling. ENSURE that the main gas valve is off before leaving the laboratory. Curriculum Objectives 8-3-10 Explain, using the particle theory of matter, the relationships among pressure, volume, and temperature of liquid and gaseous fluids. GLO: A2, D4 8-3-05 Plan and conduct experiments to determine factors that affect flow within a given system. Examples: temperature, pressure, tube diameter... GLO: C1, C2, D3, E2 8-0-1a Formulate specific questions that lead to investigations. Include: rephrase questions to a testable form; focus research questions. GLO: A1, C2 (ELA Grade 8, 3.1.2; Math: SP-I.1.8) 8-0-3a Formulate a prediction/hypothesis that identifies a cause and effect relationship between the dependent and independent variables. GLO: A2, C2 (Math: SP-I.1.8) 8-0-3b Identify the independent and dependent variables in an experiment. GLO: A2, C2 8-0-3c Create a written plan to answer a specific question. Include: apparatus, materials, safety considerations, steps to follow, and variables to control. GLO: C2 (ELA Grade 8, 3.1.4) 8-0-4a Carry out procedures that comprise a fair test. Include: controlling variables, repeating experiments to increase accuracy and reliability. GLO: C2 8-0-4e Demonstrate work habits that ensure personal safety, the safety of others, and consideration for the environment. Include: keeping an uncluttered workspace; putting equipment away after use; handling glassware with care; wearing goggles when required; disposing of materials safely and responsibly. GLO: C1 8-0-5a Make observations that are relevant to a specific question. GLO: A1, A2, C2 8-0-5c Select and use tools to observe, measure, and construct. Include: microscope, concave and convex mirrors and lenses, chemical indicators. GLO: C2, C3, C5 8-0-5e Estimate and measure accurately using SI and other standard units. Include: determining volume by displacement of water. GLO: C2, C5 (Math: SS-IV.1.6, SS-III.1.5, Math: SS-III.1.6, SS-I.1.5) 8-0-5f Record, compile, and display observations and data, using an appropriate format. GLO: C2, C6 (ELA Grade 8, 3.3.1; Math: SP-III.2.8) 8-0-6b Interpret patterns and trends in data, and infer and explain relationships. GLO: A1, A2, C2, C5 8-0-6c Identify strengths and weaknesses of different methods of collecting and displaying data, and potential sources of error. GLO: A1, A2, C2, C5 (ELA Grade 8, 3.3.3) 8-0-6f Identify how the original plan evolved and justify the changes. GLO: C2, C3 (ELA Grade 8, 3.3.4) 8-0-7a Draw a conclusion that explains investigation results. Include: explaining the cause and effect relationship between the dependent and independent variables; identifying alternative explanations for observations; supporting or rejecting a prediction/hypothesis. GLO: A1, A2, C2 (ELA Grade 8, 3.3.4) 8-0-7b Critically evaluate conclusions, basing arguments on fact rather than opinion. GLO: C2, C4 8-0-7c Identify a new prediction/hypothesis based on investigation results. GLO: A1, C2 (ELA Grade 8, 3.3.4) 8-0-7f Reflect on prior knowledge and experiences to construct new understanding and apply this new knowledge in other contexts. GLO: A2, C4 (ELA Grade 8, 1.2.1) Shannon DeSmet Tammy Dudenhoffer Teacher Modeling: Getting to Know the Bunsen Burner Handout: Parts of the Bunsen Burner: Label the Bunsen burner as we discuss the parts as a class: Word Bank: air hole barrel base gas tubing hottest part of flame inner blue cone of flame outer, non-luminous flame regulating collar Bunsen Burner Manipulation When teaching the class how to manipulate a Bunsen burner flame, have each group of students with their own Bunsen burner. The Bunsen burners should not be lit and for safety reasons should have no burner hose. This way, while you are teaching the class to manipulate the Bunsen burner, they can identify and move the parts along with you. Shannon DeSmet Tammy Dudenhoffer 1. Move the gas control needle valve to control the height of the flame. 2. Estimate the temperature the substance must be heated at, according to the procedure: - to make the flame burn hotter and larger, open the air mixture valve accordingly. - to make the flame burn cooler and smaller, close the air mixture valve accordingly. When the flame is properly adjusted, a slight buzzing sound will be audible. This is and example of a well-adjusted burner flame And this is an example of poorly adjusted burner flame Lighting the Bunsen Burner: After completing the safety inspection, the following steps may be followed to light the burner: 1. Connect the burner hose to the gas outlet. The gas outlet handle should be in the fully closed position with the handle at a right angle to the outlet pipe. 2. Turn the gas valve on the gas outlet to the fully open position (handle is now parallel to gas outlet). 3. With the main gas valve fully open, open the gas adjustment at the base of the burner by several turns to give good gas flow through the burner. 4. Light the burner using a sparkler or lighter to the side of the mouth of the burner. 5. Adjust the amount of air in the flame by turning the barrel of the burner. A flame with too little air mixed in it has a light blue appearance and more air must be mixed in by unscrewing the barrel to the left. Types of Flames: Shannon DeSmet Tammy Dudenhoffer A Bunsen burner can produce 3 different types of flames: 1. Safety Flame - The "coolest" flame is a yellow / orange color. It is approximately 300°C. It is never used to heat anything, only to show that the Bunsen burner is on 2. Blue Flame – a. k. a. invisible flame or medium flame. It is difficult to see in a well-lit room and is the most commonly used flame. It is approximately 500°C. 3. Roaring Blue Flame - The hottest flame, characterized by a light blue triangle in the middle and it is the only flame of the 3 which makes a noise. It is approximately 700°C. Students should now practice manipulating the gas control and the oxygen control to reproduce these three types of flames. Once this is completed answer the following questions. Question #1: What happens to the color of the flame if you open the air mixture valve wide open? Question #2: What would you do to the air mixture and gas valve to get the highest flame possible? Question #3: What do you do to the air mixture valve and gas valve to get the hottest flame (Roaring blue flame)? Shannon DeSmet Tammy Dudenhoffer Scientific Inquiry Using the Skill: Inquiry Question: What is the relationship between pressure, volume, and temperature when a liquid is compressed or heated and a gas is compressed or heated? EXPERIMENT 1 - HOW DOES TEMPERATURE EFFECT THE VOLUME OF A GAS, AND WHY? Problem How does temperature affect the volume of a gas, and why is this so? Hypothesis Explain what you think will happen to the volume of the gas once it is heated and justify your answer using the particle theory. Materials: • 250 mL flask • glass tubing • 2 – hole rubber stopper • colored water • thermometer • marking pen • safety glasses Safety Precautions Wear safety goggles during this activity. Follow your teacher’s instructions for inserting the thermometer and glass tubing into the stopper. Apparatus Procedure 1. Read the procedure that follows. Then use the particle theory to predict the results. Write your prediction in your notebook. Shannon DeSmet Tammy Dudenhoffer 2. Draw a table in your notebook with two columns. Title them “Slug Position” and “Temperature”. 3. Set up the apparatus as shown. Your teacher will show you how to put the glass tubing and thermometer through the rubber stopper without breaking them or hurting yourself. The glass tubing and thermometer should reach halfway into the flask. The slug of colored water should be about 5 mm long. At the start of the activity it should be just above the stopper. Your teacher will tell you how to get it there if you have trouble. Mark the position of the slug. 4. Warm the flask gently. Do so by wrapping your hands around it for a few minutes. Mark the new position of the slug of water. Record the temperature. Then record the position of the slug above the stopper. 5. Light the Bunsen burner using the methods discussed in the pre lab when learning how to manipulate a Bunsen burner. 6. Warm the flask still more by moving it over a Bunsen burner with a Blue flame. Touch the flask from time to time so you won’t overheat it. (*Caution - The thermometer will break if the temperature goes above its range.) Continue marking the positions of the slug for 3 or 4 more temperatures. Do so until the temperature is in the 70ºC – 80 ºC range. 7. Observe the slug of water as the flask cools. Analysis and Conclusions Answer the following question in your notebook 1. How did the slug of water move when the air was heated? 2. How did the slug of water move when the air was cooled? 3. What is the relationship between the temperature and volume of a gas? How does this agree with your prediction? 4. Explain your conclusion using the particle theory. 5. Does the particle theory suggest that your conclusion may be true for gases other than air? Explain. 6. How has this activity increased your confidence in the particle theory? EXPERIMENT 2 - EFFECT OF TEMPERATURE ON THE VOLUME OF A LIQUID Problem How does temperature affect the volume of a liquid, and why is this so? Hypothesis Explain what you think will happen to the volume of the liquid once it is heated and justify your answer using the particle theory. Materials: • 1000 mL beaker • adjustable clamp • 2-hole rubber stopper • water, glycerin, ethylene glycol • thermometer (-10ºC to 110ºC) • marking pen Shannon DeSmet Tammy Dudenhoffer • 250 mL flask • glass tubing (50 cm) • ring stand, iron ring, wire gauze • Bunsen burner • graph paper Safety Precaution Wear safety goggles during this activity. Follow your teacher’s instructions for inserting the thermometer and glass tubing into the stopper. Procedure 1. Prepare a data table with the column headings “Position of water” and “Temperature”. 2. Using the same apparatus as in Experiment 1 record the temperature, mark the position of the liquid, and record the position (the distance above the stopper) before you begin heating. 3. Using the Bunsen burner with a Blue flame begin heating the liquid slowly. Record the position of the liquid at the following temperatures - 30ºC, 35ºC, 40ºC, 45ºC, 50ºC, 55ºC, 60ºC, 65ºC. Do not heat the liquid beyond 65ºC. Analysis and Conclusions Answer the following questions in your notebook 1. What is the relationship between the temperature and volume of a liquid? 2. How does this agree with your prediction? 3. Use the particle theory to explain the results of this activity. 4. How has this activity increased your confidence in the particle theory? Resources: Lab Activity http://www.oecta.on.ca/curriculum/structures/grade8/8sgst3b.pdf - grade 8 Science Safety from Manitoba Curriculum http://www.edu.gov.mb.ca/k12/docs/support/scisafe/appendixb.html Safety precautions http://www.med.cornell.edu/ehs/updates/bunsen_burner_safety.htm Bunsen burner adjustments http://www.uwplatt.edu/chemep/chem/chemscape/labdocs/catofp/bunsbur/adjust.htm and http://www.fordhamprep.org/gcurran/sho/sho/skills/burner.htm