What Happens to Create the Lode?
advertisement

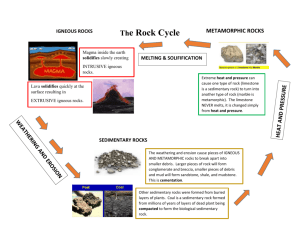
What Happens to Create the Lode? Original lesson by Stephen Fuller Modified and adapted by Karen Munroe & Rachel Hughes Time: 1 class period Preparation 1 hour Time: Materials: 3 beakers per group, ammonia, iron acetate, alum, Epson salts., steel wool, vinegar, plastic spoons, student worksheet Abstract As a result of this laboratory experience students will be able to explain how solutions precipitate out in different conditions and compare this to the formation of ore deposits in hydrothermal systems. Objectives Students will: 1. Understand how mineral deposits are formed and why they are not evenly dispersed. 2. Create and describe 3 different precipitates from four solutions simulating mineral ore deposit formation in sedimentary rock. National Science Education Standards: CONTENT STANDARD B Physical Science CHEMICAL REACTIONS Chemical reactions can take place in time periods ranging from the few femtoseconds (10-15 seconds) required for an atom to move a fraction of a chemical bond distance to geologic time scales of billions of years. Reaction rates depend on how often the reacting atoms and molecules encounter one another, on the temperature, and on the properties--including shape--of the reacting species. Teacher Background Igneous, metamorphic and sedimentary rocks are the three main types of rock. Sedimentary rock is formed in three main ways—by the accumulation of other rocks pieces (known as 'clastic' sedimentary rocks), by the accumulation of biogenic activity (fossils), and by precipitation from solution. As sediment deposits build up, the overburden (or lithostatic) pressure squeezes the sediment into layered solids in a process known as lithification ("rock formation"). Sedimentary rocks contain important information about the history of the earth. They contain fossils, the preserved remains of ancient plants and animals. The composition of sediments can also provide clues to the original igneous rock. Differences among successive layers of sedimentary rock indicate changes to the environment which have occurred over time. Sedimentary rocks can contain fossils because, unlike most igneous and metamorphic rocks, they form at temperatures and pressures that do not destroy fossil remnants. Sedimentary rocks include common types such as chalk, limestone, sandstone, clay and shale. Sedimentary rocks cover 75% of the earth's surface, but the total contribution of sedimentary rocks is estimated to be only 5% of the total. As such, the sedimentary sequences we see represent only a thin veneer over a crust consisting mainly of igneous and metamorphic rocks. The process of precipitation of solids as two solutions meet is critical to the creation of mineral deposits. Gold deposits are formed by a very wide variety of geological processes. The majority of primary gold deposits fall into two main categories: lode gold deposits or intrusion-related deposits. Lode gold deposits are generally high-grade, thin, vein- and fault-hosted. They are comprised primarily of quartz veins also known as lodes or reefs. Lode-gold deposits are intimately associated with tectonic plate collision events within geologic history. Most lode gold deposits are sourced from metamorphic rocks because it is thought that the majority are formed by dehydration of basalt during metamorphism. The gold is transported up faults by hydrothermal waters and deposited when the water cools too much to retain gold in solution. Intrusive related gold is generally hosted in granites, porphyry or rarely, dikes. Intrusive related gold usually also contains copper, and is often associated with tin and tungsten, and rarely, molybdenum, antimony and uranium. Intrusive-related gold deposits rely on gold existing in the fluids associated with the magma, and the inevitable discharge of these hydrothermal fluids into the wall-rocks. Related and Resource Websites www.mii.org/pdfs/naturestorehouse.pdf Activity Note: make the iron acetate 5 days in advance of the lesson. Take a jar with a lid, label it iron acetate, and fill it one-half full with steel wool. Add enough vinegar to cover the steel wool. Secure the lid on the jar and allow the jar to stand undisturbed for 5 days. Explain to your students the three major types of rock and how they are formed. Emphasize the importance of sedimentary rock and the mineral deposits that can be formed within them. Today’s exercise is going to simulate an aqueous solution from a hydrothermal system coming in contact with three other aqueous solutions The household ammonia is the one common solution. You can label it as the main solution. The students are going to mix this with 3 other chemicals. Procedure: Precipitate 1: Fill the beaker half full with water. Add 1/2 teaspoon alum to the water and stir. Stir in 2 tablespoons of ammonia. CAUTION: ammonia has strong fumes, be careful not to breathe the fumes. Allow the solution to stand for 5 minutes. Observe the formation of the aluminum hydroxide precipitate. As it sits, the precipitate will settle to the bottom of the jar. Precipitate 2: Fill the beaker half full with water. Add 1 teaspoon of Epsom salt to the water and stir to dissolve. Pour 2 teaspoons of ammonia into the jar. DO NOT STIR. Allow the solution to stand for 5 minutes. Observe the formation of the magnesium hydroxide precipitate as the ammonia mixes with the Epsom salt solution. As it sits, the precipitate will settle to the bottom of the jar. Precipitate 3: In a beaker place a tablespoon of liquid from the jar labeled iron acetate. Add one tablespoon of ammonia and stir. A dark green substance should form immediately. Recap the experiment and explain that this process is occurring underground and in hydrothermal vents right now and in the past. Explain that this is why we have mineral ore deposits and why they are not evenly spread around the globe. Extensions: 1.) Explain and calculate the stoichiometry of the three reactions. Have students balance the equation and calculate the amount of product produced. Embedded Assessment Students make scientific observations on the attached worksheet.