The Pennsylvania State University College of Medicine Biological
advertisement

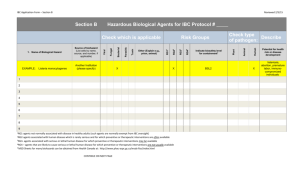
The Franklin and Marshall College Institutional Biosafety Committee BSL2 Biosafety Review: Research Registration Form 1. Instructions PRIOR TO BEGINNING, MAKE CERTAIN YOU ARE USING THE CORRECT VERSION OF THIS FORM BY DOWNLOADING THE LATEST VERSION FROM THE BIOSAFETY WEB SITE. Information must be provided in all boxes shaded yellow as well as in all other appropriate tables. If you reply ‘Yes’ to a question, you need to provide additional details in the table associated with that question. Incomplete forms will be returned without being processed. Examples of information to supply in a table are given in cells shaded grey in the table. Use unshaded cells for your information. You must also electronically sign (that is, type your signature) this BSL2 Biosafety Review form, which includes the Acknowledgement of Your Responsibilities, prior to submission. Since the IBC includes Institutional and community members from a variety of backgrounds, it is essential to provide information in a manner that is understandable to readers who are scientifically educated but not specialists. Table 1 PI Information PI name e-mail address Department Previous IBC protocol no. (PI name and date of last submission) Guidance for assigning biosafety levels (BL) can be found at http://www.cdc.gov/biosafety/publications/index.htm (BMBL, 5th ed.) and http://oba.od.nih.gov/rdna/nih_guidelines_oba.html (NIH Guidelines). Standard Operating Protocols (SOPs) for working with the materials registered in this Form must be submitted with this form and maintained in your laboratory. Additional SOPs for your lab may need to be developed depending on the materials registered in this BSL2 Biosafety Review Form. See template documents (available on the Biosafety web site) for suggestions of what to include in SOPs and examples of SOPs. These template SOPs can be modified as appropriate for working with the specific agents you are registering and for your lab. 2. Project Title and Description of Work Provide a brief title describing the work encompassed by the biohazards being registered and a short (usually less than 150 words) summary in lay terminology of 1) the overall goal of the research and 2) the planned research activities to assist Committee members to understand how the registered materials will be manipulated. This description must be written in lay scientific terms. Table 2. Project Title and Description of Work 2A: Project Title 2B: Summary including overall goal(s) and description of research activities (in lay scientific terminology) 1 3. Unfixed Materials from Humans or Non-Human Primates 3-i. Does this work involve any unfixed materials derived from humans or non-human primates? These materials include established cell lines, blood, other fluids, tissues, and primary cells. Yes No If no, go to item 4. If yes, 3a. Specify unfixed human or non-human primate material(s) employed including specific name(s) or description(s) and Institutional Review Board (IRB) approval number as appropriate in Table 3. (Note that work with any unfixed human-derived material is at least BSL2.) 3b. Forward the SOP(s) for work with these materials in your lab to the Biosafety Officer with the completed BSL2 Biosafety Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the material. Table 3. Unfixed Human or Non-Human Primate Materials Human or primate materials Human cell lines E Example Example Human body fluids Comments (e.g. primary tissue, established cell line, known pathogens) HEK293 cell line and BxPC-3 pancreatic adenocarcinoma cell line Including, but not limited to, blood and saliva collected from volunteers BL IRB # SOP ID# BL2 NA RLK #1 BL2 78901EP Infection Control Policy V-1; Standard Body Substance Precautions (SBSP) (Add additional rows as necessary to the table above.) 3-ii. Will any federally funded work with human embryonic stem cells (hESC) and/or human induced pluripotent stem cells (hiPSC) be conducted? Yes No If no, go to item 4. If yes, contact the Biosafety Officer; additional information will be required. 4. Biological Toxins or Carcinogens 4-i. Does this work involve toxins of biological origin? Yes No If no, go to item 4-ii. If yes, 4a. Specify these toxin(s) in Table 4 below. 4b. Forward the SOP(s) for work with these materials to the Biosafety Officer with the completed BSL2 Biosafety Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the material 2 Table 4. Toxins of Biological Origin Toxin Example Comments (include dose, LD50, route of administration, safety precautions, etc.) 25 ng/kg mouse body weight (human LD50 = 100 ng/kg); IP injection; All personnel handling the toxin have received booster vaccinations. Rooms will be posted while DT is administered and animal cages will be marked accordingly. Diphtheria toxin SOP ID# RLK #2 (Add additional rows as necessary to the table above.) Yes 4-ii. Will toxins, carcinogens, or hazardous chemicals be used in animals? No IACUC protocol number(s) (if available) If no, go to item 5. If yes, contact the Biosafety Officer and IACUC; additional forms will be required. If you have not yet submitted an IACUC protocol, write ‘to be submitted’ in this column. 5. Infectious Agents that do not contain recombinant DNA (recombinant infectious agents should be listed in Item 6) 5. Does your work involve any non-recombinant agents that are potentially infectious, including viruses, bacteria, or parasites? Note: recombinant infectious agents (that is, containing recombinant DNA) belong in Item 6 and Table 6 rather than in Item 5. Yes No If no, go to item 6. If yes, 5a. Specify agent(s) employed and indicate the BSL and/or Risk Group (RG) of each if applicable in Table 5 (a useful web link for help in determining BSL and/or RG is http://www.absa.org/riskgroups/index.html). 5b. Forward the SOP(s) for work with agent(s) in your lab to the to the Biosafety Officer with the completed BSL2 Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the agent. Table 5. Infectious Agents Infectious agent Example Vaccinia virus Comments (e.g. strain, modifications) BL BL2+ Western Reserve strain RG 2 (Add additional rows as necessary to the table above.) 6. Recombinant Infectious Agents 6. Does this work involve recombinant infectious agents including viruses, bacteria, or parasites? Yes No If no, go to item 7. 3 SOP ID # EXAMPLE RLK #3 If yes, 6a. In Table 6a list recombinant pathogens including bacteria, parasites, and viruses (including recombinant retroviruses). Include the type, specific strain, or name as applicable, and nature of the pathogen. 6b. In Table 6b list information about recombinant retroviral vectors (including lentiviral vectors). 6c. In Table 6c list recombinant inserts (including genes, cDNAs, microRNA or shRNA cassettes, etc.) expressed in the pathogens described in Tables 6a and 6b. Provide any additional comments that will assist Committee members in assessing biosafety concerns. 6d. Forward the SOP(s) for work with these materials in your lab to the to the Biosafety Officer with the completed BSL2 Biosafety Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the agent. Table 6a. Recombinant Infectious Agents Pathogen type, strain, or name Is this a retroviru s? Is this replication competent ? If this is replication defective, is helper virus(es) used? Yes/No No Yes/No No Yes No If YES, fill out Table 6B Example Adenovirus Cre recombinase (Ad-CMVCre) pLenti6-myc lentiviral vector Yes/No No If this is replication defective, is a packaging cell line or replicationdefective packaging plasmids used? Yes/No Yes Does this encode a product that increases expression of an oncogene or decrease expression of a tumor suppressor? Yes/No No No Yes Yes Comments (e. g., oncogene name, tumor suppressor targeted by insert, means used to make virus replication defective, helper virus ID) SOP ID # BL Deleted for the essential E1 gene as well as the E3 gene; thus, this agent is non-replicative Human c-myc oncogene; lentiviral particles produced are replication defective & only contain the c-myc gene RLK# 1 BL2 RLK# 3 BL2+ (Add additional rows as necessary to the table above.) Table 6b. Recombinant Retroviruses Name of vector or system Example ViraPower System Source (e.g., company, or scientist) Invitrogen ‘Generation’ or number of plasmids in system 3rd generation; 4 plasmids (Add additional rows as necessary to the table above.) 4 Envelope gene(s) encoded VSV-G Tropism Pantropic Can the expression vector infect human cells? Yes/No SOP ID# Yes EXAMPLE RLK #3 Table 6c. Recombinant DNA in Infectious Agent(s) that are Listed in Tables 6a and 6b Examples Nature of insert (e.g., gene, cDNA, microRNA, shRNA cassette) Gene or product name (or class of product) Source/species Biological role of rDNA Biologically active protein(s) or product(s) Does product potentially initiate or promote oncogenesis? Yes/No Recombinant pathogen (from Tables 6a and 6b) in which this will be present Cells to receive rDNA during experiment (e.g. HeLa, human, mouse) NIH category (III-A to III-F) BL SOP ID # Insert Insert Gene Gene c-Myc Cre Human Phage P1 Encodes transcription factor c-myc protein Yes Site-specific recombination ViraPower lentivirus vector Human HEK293 Ad-CMV-Cre III-D BL2+ RLK #3 III-D BL2 RLK #2 Insert 1 Insert 2 Insert 3 Insert 4 Cre No Human HEK293 (If more genes need to be listed, duplicate this table and paste it below this original table.) 7. Recombinant DNA (not including recombinant viruses and other infectious agents, which were listed in Tables 6a and 6b) 7. Does this work involve recombinant DNA other than recombinant infectious agents (which were addressed in Tables 6a and 6b)? Yes No If no, go to item 8. If yes, 7a. List recombinant inserts (including genes, cDNAs, microRNA or shRNA cassettes, etc.) used in Table 7. For assistance, the NIH guidelines are available at http://oba.od.nih.gov/rdna/nih_guidelines_oba.html. 7b. Forward the SOP(s) for work with these materials in your lab to the Biosafety Officer with the completed BSL2 Biosafety Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the material. 5 Table 7. Recombinant DNA Examples Insert Nature of insert (e.g., gene, cDNA, microRNA, shRNA cassette) Gene or product name (or class of product) Source/species Biological role of rDNA Biologically active protein(s) or product(s) Does product potentially initiate or promote oncogenesis? Yes/No Original host/vector (e.g. E.coli/pUC; insect cells/baculovirus) Cells to receive rDNA during experiment (e.g. E. coli, yeast, HeLa) NIH category (III-A to III-F) BL SOP ID # Insert 1 Insert 2 Insert 3 Insert 4 Gene Various amino acid transporter genes Yeast S. cerevisiae Encodes amino acid transporters Amino acid transporters No E. coli pBR derivatives that also replicate in yeast E. coli and yeast (S. cerevisiae) III-F BL1 EXAMPLE RLK #4 (If more genes need to be listed, duplicate the table above and paste it below this original table.) 8. Biohazards in Animals 8-i. Will any of the biohazards or recombinant DNAs described above (items 3-7; including unfixed humanderived materials) be used in animals? Yes No If no, go to item 8-ii. If yes, specify biohazard(s)/recombinant DNAs, animal specie(s) and Institutional Animal Care and Use Committee (IACUC) protocol number(s) in Table 8a. If you have not yet submitted an IACUC protocol, write ‘to be submitted’ in this column. Table 8a. Biohazards in Animals Biohazard/recombinant DNA Example BxPC-3 human cells Animal Specie(s) and description Nude mice (Add additional rows as necessary to the table above.) 6 Comments BL Prior to injection in mice, BxPC-3 cells will be transfected with plasmid pLWW, which expresses GFP. BL2 IACUC protocol # 20YY-ZZZ 8-ii. Will any experiments, with or without biohazards, be conducted in genetically manipulated animals (e.g., transgenic or knockout animals)? Yes No If no, go to item 9. If yes, 8b. Provide appropriate information in Table 8b. 8c. Forward the SOP(s) for work with these materials in your lab to the Biosafety Officer with the completed BSL2 Biosafety Review form for IBC review. Provide an identification number (SOP ID #) that permits matching the SOP to the material. Table 8b. Genetically Manipulated Animals Gene altered; Transgene (TG) or knockout (KO) and Source Example Simian diptheria toxin receptor (TG) Species of gene/marker Monkey Species and type of genetically altered animal Mouse Biohazard (if used) Does change initiate or promote oncogenesis ? Yes/No BL IACUC protocol # No 2+ 20YY-ZZZ Vaccinia virus (Add additional rows as necessary to the table above.) 9. Biohazards in Humans 9. Will any of the biohazards or recombinant DNAs described above (items 2-6) be used in humans? Yes No If no, go to item 10-i. If yes, specify biohazard(s) and IRB protocol number(s) in Table 9. If recombinant DNA is used, the documents pertaining to the review by the NIH RAC must also be sent to michael.billig@fandm.edu. Table 9. Biohazards or Recombinant DNA in Humans Biohazard or recombinant DNA Example Recombinant adenovirus Comments Trial Phase 1 x 106 adenovirus with tumor suppressor gene p53 will be injected into hepatic artery feeding hepatoma lesion, as per protocol xx-xxx (attached) I (Add additional rows as necessary to the table above.) 7 IRB protocol # FDA New drug appro val # (IND) 78902 pending WWW For protocols with recombinant DNA or derived materials, list NIH category and NIH RAC protocol # and status NIH category III-C; NIH RAC #0311-WWW; public review done; summary attached Informed consent attached; required Yes 10. Facilities 10-i. Will experiments with any of the above biohazards be conducted in your laboratories? Yes No If no, go to item 10-ii. If yes, list your laboratory rooms in which experiments with any of the above materials will be conducted in Table 10a, including the assigned BSL rating for the room and the appropriate equipment available for work with these materials [e.g., BSL2 work generally requires certified biosafety cabinets (include the date of the most recent certification, which must be within the last 12 months), appropriate safeguards for aerosol-generating procedures such as centrifugation, vortexing, sonication, pipetting, etc., facilities for appropriate decontamination]. Table 10a. Your Laboratories and Facilities Room # Example C57WW Assigned BL Equipment for safe conduct of work with biohazards BL2+ EXAMPLE Two biosafety cabinets, centrifuge with closed buckets, sharps containers in lab, biohazard warning sign on door, SOPs available in lab notebook, personal protective equipment including lab coats, gloves, & face protection utilized, autoclave available on the same hallway, both red and orange trash bags available for use with the different levels of biohazards as appropriate Biosafety Cabinet Yes/No Yes two If Yes, date certified Both certified on 3/21/YY (Add additional rows as necessary to the table above.) 10-ii. Will other facilities, labs, or equipment in other labs be used for work with any biohazard(s) listed above? Yes No If no, go to item 11. If yes, list additional facilities, common equipment rooms or labs of other faculty, within the institution that will be used for any biohazard(s) in this registration in Table 10b. If this work includes animals, you must indicate the rooms in the animal facilities that will be used. The status of this protocol will be copied to the supervisor of the facility/room. Table 10b. Other Facilities and Labs Used for Work Facility & Room # Example Examples CAQ WWW GCRG Facility/room supervisor & e-mail address Assigned BL Penny Devlin pdevlin@hmc.psu.edu Rebecca Jenkins rjenkins@hes.hmc.psu. edu 2 JCAHO certified Biohazard Human cells in nude mice Human blood; recombinant adenovirus Equipment utilized and safety measures including cleaning/decontamination methods Cells in double, sealed, shatterproof containers when transported to CAQ Standard precautions as per routine; contact isolation for work with recombinant virus (Add additional rows as necessary to the table above.) 11. Internal Transfers 11. Will any biohazard(s) listed above be transported between rooms within the Institution? Yes No If no, go to item 12. 8 Approval for use of this facility? Yes/No Approval ID Yes 20YY-ZZZ Yes WW-YY If yes, in Table 11 list each biohazard that will be transported and detail means and precautions utilized including packaging of materials during transport. At a minimum, these materials need to be in two sealed, shatterproof containers. While gloves should be worn when working with these materials in the lab, it is not appropriate to wear gloves when transporting them through public areas. Table 11. Transport Example Biohazard Human cells Transportation safeguards Cells in double, sealed, shatterproof containers (e.g., a tube and a Tupperware container) when taken to CAQ; however, gloves are not worn in public areas during transport (Add additional rows as necessary to the table above.) 12. External Transfers 12. Will any biohazard(s) listed above be shipped or transported off campus? Yes No If no, go to item 13. If yes, in Table 12 detail 1) how each biohazard will be shipped or transported and 2) training or certification for this. Table 12. Shipping Example Biohazard Human cells Shipping safeguards Appropriate classification, packaging and labeling as per e.g. DOT, IATA, etc. regulations Training/certification Online training completed by Dr. I. Ship who is responsible for shipping these items (Add additional rows as necessary to the table above.) 13. Training BSL2 and Bloodborne Pathogen Training is required of everyone working in a designated BSL2 laboratory, even if the individual’s work only involves BSL1 agents and manipulations. BSL2 and Bloodborne Pathogen Training is available as a seminar offered at least 3 times a year – usually at the beginning of the semester (Contact the Biosafety Officer (Debra Frielle, X4600, dfriellefandm.edu) for information). Training is also available as a Blackboard course (requires enrollment and an exam, contact the Biosafety Officer) and as one-on-one training, by request. BSL2 training must be completed once; annual renewal of Bloodborne Pathogen training is required by OSHA. Biosafety and Bloodborne Pathogen training must be documented in writing; documentation will be maintained by the Biosafety Officer. If you wish, copies of the training documentation may be requested from the Biosafety Officer and maintained in your lab as well. In addition, you must have completed all appropriate institutional Chemical Safety Training within the last year. EHS provides the annual Chemical Lab Safety training (contact Denise Freeman, X4153, denise.freeman@fandm.edu) and maintains documentation of training. 13. Have you been appropriately trained to work with each of the above biohazards? Yes No If yes, provide details of your training such as years of experience working with each biohazard, specific training or courses, publications using the biohazards listed, or other relevant preparation in Table 13. If no, detail how you will acquire appropriate training such as mentoring (provide e-mail address for mentor) or other training in Table 13. 9 Table 13. Your Training TABLE 13A: Training for working with the biohazards listed in this form TABLE 13B: If training was not completed at F&M, where is written documentation of your training maintained? TABLE 13C: Date of completion of your most recent: Chemical Safety (must be within last year): Bloodborne Pathogens Training (must be within last year, if appropriate) Biosafety Level 2 Training 14. Supervision of Others 14-i. ‘Electronic’ (typed) initials As PI, I understand it is my responsibility to ensure that ALL new individuals who enter my lab to work with the materials registered on this Form 1) complete and document all appropriate trainings (e.g., Biosafety Training, BBP training, lab-specific training) and 2) complete documentation of any available safeguards (e.g., vaccinations, serum banking) required for the biohazards they will work with. 14-ii. Will others work with any of the above biohazards in your lab or under your direction? Yes No If yes, provide appropriate information in the Table 14a. Biosafety and Bloodborne Pathogen training of all individuals listed in Table 14a related to the biohazards specific to this protocol must be documented and signed. In addition, labspecific technique training must be completed under the supervision of the PI. Documentation of this “hands-on” training must be forwarded to the Biosafety Officer. All training documentation will be maintained by the Biosafety Officer. In addition, each person listed must complete all required annual renewals, such as Chemical Safety Training and Bloodborne Pathogens Training, that is appropriate for their activities (see list under Item 13 above). NOTE: All information for each person MUST be provided. Table 14a. Training of Personnel Name Position Biohazard(s) trained for Example Examples Dr. J. Doe, MD HMC resident also working in COM lab Human materials F. E. Smith COM grad student Recombinant DNA; vaccinia, diptheria toxin Various medical professionals (e.g., RNs, MDs) in GCRC Human materials 10 Date(s) Institutional training completed (must be within last year) REQUIRED COM Safety: 3/21/WW COM BBP*: 3/21/WW HMC Safety: 3/21/WW HMC BBP**: 3/21/WW COM Safety: 3/21/WW COM BBP*: NA HMC Safety: NA HMC BBP**: NA COM Safety: NA COM BBP*: NA HMC Safety: various dates HMC BBP**: various dates Chem Safety: BBP: BSL2: Chem Safety: BBP: BSL2: Chem Safety: BBP: BSL2: If any specific safeguards (e.g., vaccinations, serum banking) are recommended by the ‘Biosafety in Microbiological and Biomedical Laboratories’ (can be downloaded from http://www.cdc.gov/biosafety/publications/index.htm or the Advisory Committee for Immunization Practices (http://www.cdc.gov/vaccines/pubs/ACIP-list.htm#comp) for individuals working with any of the biohazards registered on this form, provide appropriate information in Table 14b. One example of a Specific Safeguard is that anyone working with unfixed human-derived materials must have documentation of vaccination or declination of vaccination for hepatitis B. Vaccinations can be obtained through Appel Health Services (X4082) at no cost to the employee, student or lab. Take the ‘Vaccine Administration Consent Form’ (available through a link at http://www.fandm.edu/ehs/bloodborne-pathogens) to your appointment. Documentation of declination of vaccination can be obtained by filling out the ‘Hepatitis B Vaccine Declination Form’ (available through a link at http://www.fandm.edu/ehs/bloodborne-pathogens). The completed form should be sent to Human Resources. DO NOT reveal personal health information in Table 14b. You only need to indicate that the decision about the safeguard(s) is appropriately documented. Table 14b. Specific Safeguards Name Position Biohazard(s) Specific safeguard(s) (e.g., vaccinaitions) TO ADHERE TO HIPAA, ONLY STATE THAT “VACCINATION FOR… OR DECLINATION OF THIS VACCINATION IS DOCUMENTED” Example Examples Dr. J. Doe, MD HMC resident also working in COM lab Human materials Vaccination for hepatitis B or declination of this vaccination is documented F. E. Smith; COM grad student Vaccinia & diptheria toxin Various medical professionals (e.g., RNs, MDs) in GCRC Human materials Vaccination for vaccinia virus within last 10 years & diptheria booster within last 10 years or declinations are documented Vaccination for hepatitis B or declination of this vaccination is documented Documentation of appropriate vaccination(s) or declination(s) REQUIRED Where documented? (Appel Health/Human Resources) Employee Health Student Health Employee Health (Add additional rows as necessary to the table above.) 15. Adverse Outcomes 15. Have there been any accidental spills, accidental exposures, or adverse outcomes with any of the above biohazards in your lab? Yes No If yes, provide details in Table 15. Table 15. Adverse Outcomes 16. Assignment of Biosafety Level Highest BSL you assigned for above biohazards Guidance for assigning biosafety levels (BL) can be found at http://www.cdc.gov/biosafety/publications/index.htm (BMBL, 5th ed.) and http://oba.od.nih.gov/rdna/nih_guidelines_oba.html (NIH Guidelines). 11 17. Acknowledgement of Your Responsibilities I the undersigned Principal Investigator recognize that I am responsible for A) developing and making accessible SOPs describing appropriate means for working with each biohazard in my lab; B) ensuring that everyone entering my lab i) is appropriately instructed and trained to work with all biohazards they handle or encounter in the lab, ii) performs all experiments with biohazards with appropriate biosafety precautions, and iii) has received any required vaccinations or other specific safeguards necessary for any biohazard they handle or encounter in the lab; C) maintaining appropriate documentation of training and safeguards for everyone in my lab who works with or encounters biohazards; D) making available to the IBC all SOPs and documentation (e.g., of training) as requested and permitting inspection of my lab by members of this Committee and other appropriate personnel; E) maintaining certification of biosafety cabinets as necessary and ensuring appropriate operation of all equipment; F) appropriately describing the biosafety concerns related to my experiments to individuals in charge of other labs or facilities in which work with these biohazards is conducted; G) immediately reporting to the Biosafety Officer (Debra Frielle, X4600; dfrielle@fandm.edu) and to EHS (Denise Freeman, X 4153, denise.freeman@fandm.edu) i) any spill of biohazardous materials or any equipment failure that could potentially result in exposure of anyone to biohazardous materials; ii) any signs, symptoms, or illnesses consistent with exposure to any biohazardous materials in my lab; H) updating this application when changes are made including adding new biohazards or new people working with biohazardous materials and reviewing this application as requested; I) ensuring that initiation of work with the biohazards being registered occurs in accordance with the appropriate sequence of submission and/or approval of this protocol as described in the ‘NIH Guidelines for Research Involving Recombinant DNA Molecules’; and J) adherence to the ‘NIH Guidelines for Research Involving Recombinant DNA Molecules’, guidelines in the current edition of the ‘Biosafety in Microbiological and Biomedical Laboratories’, and the Institutional policy by everyone working with these biohazards in my lab or under my direction. ‘Electronic’ (typed) signature of PI PI e-mail address Phone Date Table 17 PI Information Office/room number PI status (e.g., faculty, post-doc, student) 18. Willingness to Mentor (optional) Table 18. Willingness to Mentor Based on my experience and training, I am able and willing to be contacted for advice or mentorship for work with the following biosafety materials: 12 19. Notification of Others (optional) In the following table, list other individuals you want informed regarding the status of this application (e.g., lab manager, administrative assistant, etc.). Note that if the PI is not a faculty member, the faculty advisor must be listed in the table. Name Status/title e-mail address phone Office/room number (Add additional rows as necessary to the table above.) 20. Additional Comments (optional) Table 20. Additional information or comments SUBMIT THIS FORM AS A WORD DOCUMENT ALONG WITH ALL OTHER NECESSARY DOCUMENTS, SUCH AS SOPs, TO THE BIOSAETY OFFICER FOR IBC REVIEW. For F&M IBC use only Investigator & Protocol no: Date rec’d: Date approved: Version 5/2014 13