Supporting Information - Royal Society of Chemistry
advertisement
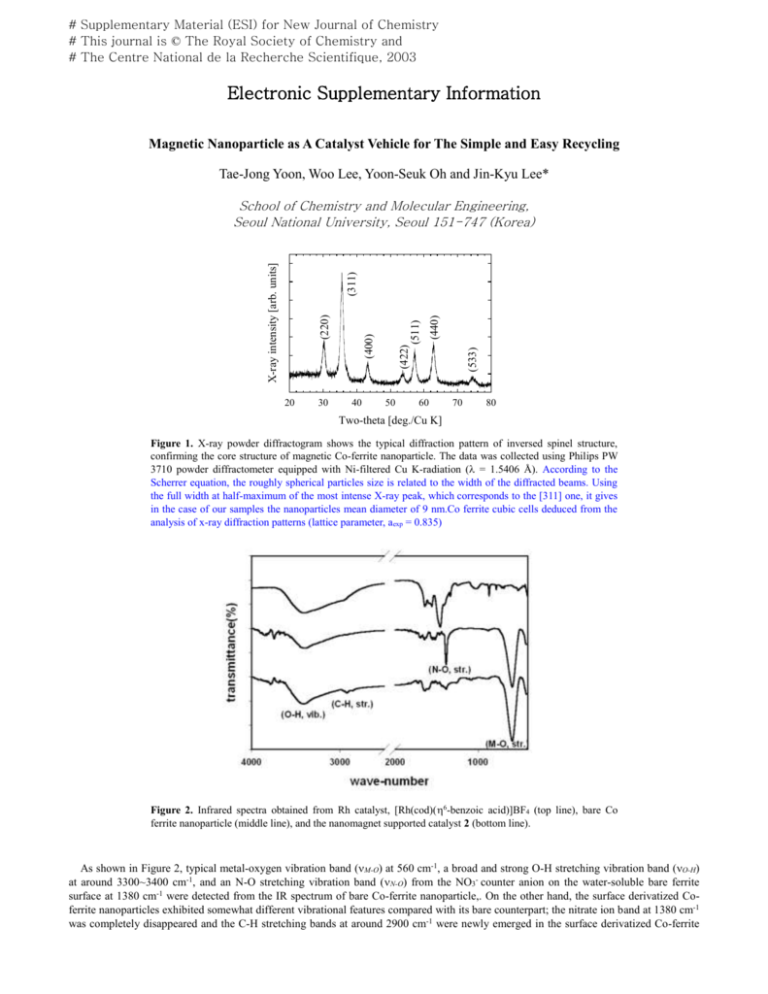
# Supplementary Material (ESI) for New Journal of Chemistry # This journal is © The Royal Society of Chemistry and # The Centre National de la Recherche Scientifique, 2003 Electronic Supplementary Information Magnetic Nanoparticle as A Catalyst Vehicle for The Simple and Easy Recycling Tae-Jong Yoon, Woo Lee, Yoon-Seuk Oh and Jin-Kyu Lee* 20 30 40 (440) (511) 50 (533) (422) (400) (220) (311) X-ray intensity [arb. units] School of Chemistry and Molecular Engineering, Seoul National University, Seoul 151-747 (Korea) 60 70 80 Two-theta [deg./Cu K] Figure 1. X-ray powder diffractogram shows the typical diffraction pattern of inversed spinel structure, confirming the core structure of magnetic Co-ferrite nanoparticle. The data was collected using Philips PW 3710 powder diffractometer equipped with Ni-filtered Cu K-radiation ( = 1.5406 Å). According to the Scherrer equation, the roughly spherical particles size is related to the width of the diffracted beams. Using the full width at half-maximum of the most intense X-ray peak, which corresponds to the [311] one, it gives in the case of our samples the nanoparticles mean diameter of 9 nm.Co ferrite cubic cells deduced from the analysis of x-ray diffraction patterns (lattice parameter, aexp = 0.835) Figure 2. Infrared spectra obtained from Rh catalyst, [Rh(cod)(6-benzoic acid)]BF4 (top line), bare Co ferrite nanoparticle (middle line), and the nanomagnet supported catalyst 2 (bottom line). As shown in Figure 2, typical metal-oxygen vibration band (M-O) at 560 cm-1, a broad and strong O-H stretching vibration band (O-H) at around 3300~3400 cm-1, and an N-O stretching vibration band (N-O) from the NO3- counter anion on the water-soluble bare ferrite surface at 1380 cm-1 were detected from the IR spectrum of bare Co-ferrite nanoparticle,. On the other hand, the surface derivatized Coferrite nanoparticles exhibited somewhat different vibrational features compared with its bare counterpart; the nitrate ion band at 1380 cm-1 was completely disappeared and the C-H stretching bands at around 2900 cm-1 were newly emerged in the surface derivatized Co-ferrite # Supplementary Material (ESI) for New Journal of Chemistry # This journal is © The Royal Society of Chemistry and # The Centre National de la Recherche Scientifique, 2003 nanoparticles, suggesting complete replacement of NO3- species from the surface of bare Co-ferrite nanoparticles by carboxylic acid functional groups in [Rh(cod)(6-benzoic acid)]BF4 complexes upon surface derivatization reaction. Experimental Section Synthesis of cationic Rh catalyst, [Rh(cod)(6-benzoic acid)]BF4: [Rh(cod)Cl]2 (0.1 g, 0.20 mmol) and AgBF4 (0.08 g, 0.40 mmol) were dissolved in 20 ml of dried EtOH. After the mixed solution was stirred for 30 min., the precipitated AgCl was filtered under N 2 and washed with dried EtOH (10 ml 2). To the combined solution of the filtrate and washed ethanol solution, benzoic acid (0.06 g, 0.50 mmol) was added. The mixed solution was stirred for 1 h. and all the solvent was removed by evaporation. The solid residue was recrystallized by acetone/diethyl ether to give pure compound 1 (0.36 g, 87 %). 1 H NMR (acetone-d6, ppm): 8.04(d, 2H, ph), 7.62(m, 1H, ph), 7.50(m, 2H, ph), 4.14(s, 4H, cod), 2.54(m, 4H, cod), 1.79(m, 4H, cod); 13C NMR (acetone-d6, ppm): 167.75, 133.82, 131.48, 130.52, 129.36, 129.30, 28.71; MS (FAB): m/z = 420[M+]. Typical procedure for the hydroformylation reaction by the nanomagnet supported catalyst: A Teflon beaker containing 10 ml of dried CH2Cl2 solution of nanomagnet supported catalyst (2, 4 mg, [Rh] = 0.018 mmol) and 4-vinylanisole (0.1 ml, 0.75 mmol) with a magnetic stirring bar, was placed in 100 ml of autoclave. The autoclave was flushed with CO gas and then pressurized to the desired pressure (170 psi). The hydrogen line was attached to the autoclave and then the pressure was gradually increased to the desired level (total pressure = 500 psi). The autoclave was placed in a pre-heated oil bath and the mixture was stirred by a magnetic stirrer. After the appropriate reaction time elapsed, the autoclave was cooled to room temperature and the excess H 2/CO gas was released. A strong NdFeB magnet was placed under the bottom of the reaction flask and let nanomagnet supported catalyst (2) aggregated on the bottom to be isolated and reused. In order to save time, a strong magnet could be covered with wrapping film and placed into the reaction flask after the reaction was completed. The decanted solution from the magnet was concentrated by vacuum evaporation and then the product was immediately analyzed by 1H NMR spectroscopy. In order to rule out the possible contribution of any free Rh complex in the reaction solution and to check the stability of the nanomagnet supported catalyst against the so-called "leaching-out" of catalyst molecules from the supporter, the actual content of metallic elements in the solution was analyzed by ICP-AES. No significant amount of Rh content over 0.002 ppm was detected from the product solutions after the nanomagnet supported catalyst was removed. FT-IR spectra of nanomagnet supported catalyst (2) before and after the catalytic reaction did not show any significant differences such as appearing a new CO stretching peak at 1800~1900 cm-1 or disappearing a C-H stretching peaks from cod (cyclooctadiene) at 2800~2900 cm-1, suggesting that the Rh complex itself attached on the nanomagnet surface, [Rh(cod)(6-benzoic acid)]+, does not changed even though some of ligands must be partially displaced with CO, H2 and olefin to play as an active catalyst during the catalytic reaction. It seems that cod and/or benzoic acid are partially detached from Rh by accommodating CO, H2, and olefin during the reaction, and returned to their original form of [Rh(cod)(6-benzoic acid)]+ after the reaction. The detailed investigation is necessary to understand the whole reaction mechanism. Based on these experimental results, however, it can be corroborated that cationic Rh complexes existing on the surface of nanomagnet Co ferrite particles prevent the nanomagnets from aggregation by providing the cationic charge centers for electrostatic repulsion, and that Rh complexes existing on the nanomagnet surface show a good stability against the “reaching-out” phenomena to be used as an immobilized recyclable catalyst in the solvent system investigated. Experiments for determining the catalytic activities of nanomagnet supported Rh-catalyst (2) towards other valuable reactions such as hydrosilylation and hydrogenation and various ionic catalyst systems anchored on the nanomagnet surface are currently under investigation.








