Rocks and Minerals
advertisement

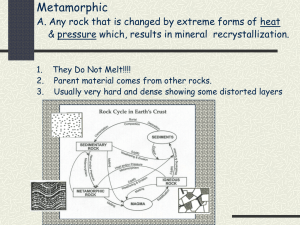
Rocks and Minerals A – B1 9. The table below shows some properties of four different minerals. 1. Which element is most abundant in Earth’s crust? (1) nitrogen (3) oxygen (2) hydrogen (4) silicon 2. Which group of elements is listed in increasing order based on the percent by mass in Earth’s crust? (1) aluminum, iron, calcium (2) aluminum, silicon, magnesium (3) magnesium, iron, aluminum (4) magnesium, silicon, calcium 3. Oxygen is the most abundant element by volume in Earth’s (1) inner core (3) hydrosphere (2) troposphere (4) crust 4. What happens to the density and temperature of rock within Earth’s interior as depth increases? (1) density decreases and temperature decreases (2) density decreases and temperature increases (3) density increases and temperature increases (4) density increases and temperature decreases The minerals listed in the table are varieties of which mineral? (1) garnet (3) quartz (2) magnetite (4) olivine 9. The basaltic bedrock of the oceanic crust is classified as (1) felsic, with a density of 2.7 g/cm3 (2) felsic, with a density of 3.0 g/cm3 (3) mafic, with a density of 2.7 g/cm3 (4) mafic, with a density of 3.0 g/cm3 10. The diagram below shows the index minerals of Mohs hardness scale compared with the hardness of some common objects. 5. The data table below shows the density of four different mineral samples. A student accurately measured the mass of a sample of one of the four minerals to be 294.4 grams and its volume to be 73.6 cm3. Which mineral sample did the student measure? (1) corundum (3) hematite (2) galena (4) quartz 6. Which mineral is the major component of drywall? (1) talc (3) muscovite mica (2) calcite (4) selenite gypsum 7. Which mineral has a metallic luster, a black streak, and is an ore of iron? (1) galena (3) pyroxene (2) magnetite (4) graphite 8. Which property is most useful in distinguishing pyroxene from amphibole? (1) sample size (3) type of luster (2) hardness (4) angles of cleavage Which statement is best supported by the diagram? (1) A fingernail will scratch calcite but not gypsum. (2) Calcite will be scratched by a copper penny. (3) The mineral apatite will scratch topaz. (4) A steel file has a hardness of about 7.5. 11. Which rock is sedimentary in origin and formed as a result of chemical processes? (1) granite (3) breccias (2) shale (4) dolostone 12. In which set are the rock drawings labeled with their correct rock types? 13. The diagram below represents geological processes that act continuously on Earth to form different rock types. 15. What is a common use for the mineral that is mined at the southern end of the two largest Finger Lakes? (1) making talcum powder (3) polishing jewelry (2) vulcanizing rubber (4) melting ice 16. The gypsum deposits in New York State were formed (1) as a result of volcanic eruptions (2) as a result of metamorphism (3) in a shallow ocean (4) in a glacial outwash plain 17. The mineral wollastonite has a hardness of 4.5 to 5. Which New York State mineral could easily scratch wollastonite? (1) garnet (3) talc (2) halite (4) gypsum Which table correctly classifies each rock type? 18. Which combination of temperature and pressure is inferred to occur within Earth’s stiffer mantle? (1) 3500°C and 0.4 million atmospheres (2) 3500°C and 2.0 million atmospheres (3) 5500°C and 0.4 million atmospheres (4) 5500°C and 2.0 million atmospheres 19. The photograph below shows an igneous rock. Base your answers to questions 14 through 17 on the map below, which shows areas where certain minerals were mined in significant amounts during 1989. What is the origin and rate of formation of this rock? (1) plutonic with slow cooling (2) plutonic with rapid cooling (3) volcanic with slow cooling (4) volcanic with rapid cooling 20. The pie graph below shows the elements comprising Earth’s crust in percent by mass. 14. In which New York State landscape region was most of the garnet mined? (1) Catskills (3) Tug Hill Plateau (2) Adirondack Mountains (4) Erie-Ontario Lowlands Which element is represented by the letter X? (1) silicon (3) nitrogen (2) lead (4) hydrogen Base your answers to questions 21 through 23 on the block diagram below, which shows a portion of Earth’s crust. Letters A, B, C, and D indicate sedimentary layers. 21. Which event occurred most recently? (1) formation of layer A (2) formation of layer D (3) tilting of all four sedimentary rock layers (4) erosion of the igneous rock exposed at the surface 22. The igneous rock is mostly composed of potassium feldspar and quartz crystals that have an average grain size of 3 millimeters. The igneous rock is most likely (1) granite (2) pegmatite (3) gabbro (4) pumice 23. Which processes produced rock layer B? (1) subduction and melting (2) uplift and solidification (3) heat and pressure (4) compaction and cementation Base your answers to questions 24 through 26 on the block diagrams of four rock outcrops, A, B, C, and D, located within 15 kilometers of each other. The rock layers have not been overturned. 24. When the rock layers at outcrops A, B, C, and D are correlated, which rock layer would be determined to be the oldest? (1) quartzite (2) marble (3) gneiss (4) sandstone 25. Which stream velocity carried only clay particles to the depositional environment where the shale formed? (1) 0.02 cm/s (2) 0.05 cm/s (3) 10 cm/s (4) 20 cm/s 26. By which process was the quartzite formed? (1) deposition of clastic sediment (2) precipitation from seawater (3) metamorphism of sandstone (4) cementation of shells 27. The diagram below shows some features in a cave. Which type of rock was chemically weathered by acidic groundwater to produce the cave and its features? (1) siltstone (2) basalt (3) quartzite (4) limestone Rocks and Minerals B2 - C Base your answers to questions 1 through 5 on the passage and cross section below, which explain how some precious gemstones form. The cross section shows a portion of the ancient Tethys Sea, once located between the Indian-Australian Plate and the Eurasian Plate. Precious Gemstones Some precious gemstones are a form of the mineral corundum, which has a hardness of 9. Corundum is a rare mineral made up of closely packed aluminum and oxygen atoms, and its formula is Al2O3. If small amounts of chromium replace some of the aluminum atoms in corundum, a bright-red gemstone called a ruby is produced. If traces of titanium and iron replace some aluminum atoms, deep-blue sapphires can be produced. Most of the world’s ruby deposits are found in metamorphic rock that is located along the southern slope of the Himalayas, where plate tectonics played a part in ruby formation. Around 50 million years ago, the Tethys Sea was located between what is now India and Eurasia. Much of the Tethys Sea bottom was composed of limestone that contained the elements needed to make these precious gemstones. The Tethys Sea closed up as the Indian-Australian Plate pushed under the Eurasian Plate, creating the Himalayan Mountains. The limestone rock lining the seafloor underwent metamorphism as it was pushed deep into Earth by the Indian-Australian Plate. For the next 40 to 45 million years, as the Himalayas rose, rubies, sapphires, and other gemstones continued to form. 1. Which element replaces some of the aluminum atoms, causing the bright-red color of a ruby? [1] 2. State one physical property of rubies, other than a bright-red color, that makes them useful as gemstones in jewelry. [1] 3. Identify the metamorphic rock in which the rubies and sapphires that formed along the Himalayas are usually found. [1] 4. During which geologic epoch did the events shown in the cross section of the Tethys Sea occur? [1] 5. What type of tectonic plate boundary is shown in the cross section? [1] Base your answers to questions 6 and 7 on the passage and photograph below. The passage describes the properties of porphyritic rocks. The photograph shows a sample of andesite rock that has a porphyritic texture. Porphyritic Rocks 6. Identify the evidence shown by the photograph that indicates Igneous rocks that have two distinctly different that two different cooling events occurred during the formation crystal sizes have a porphyritic texture. They contain of this rock. [1] large, coarse-grained crystals called phenocrysts, which are visible to the naked eye. These crystals are surrounded by fine-grained crystals called groundmass. 7. The andesite sample in the photograph has a small percentage of quartz. List three other minerals that are found in this sample. [1] Base your answers to questions 8 through 11 on the passage below. Earth’s Early Atmosphere Early in Earth’s history, the molten outer layers of Earth released gases to form an early atmosphere. Cooling and solidification of that molten surface formed the early lithosphere approximately 4.4 billion years ago. Around 3.3 billion years ago, photosynthetic organisms appeared on Earth and removed large amounts of carbon dioxide from the atmosphere, which allowed Earth to cool even faster. In addition, they introduced oxygen into Earth’s atmosphere, as a by-product of photosynthesis. Much of the first oxygen that was produced reacted with natural Earth elements, such as iron, in the lithosphere and produced new varieties of rocks and minerals. Eventually, photosynthetic organisms produced enough oxygen so that it began to accumulate in Earth’s atmosphere. About 450 million years ago, there was enough oxygen in the atmosphere to allow for the development of an ozone layer 30 to 50 kilometers above Earth’s surface. This layer was thick enough to protect organisms developing on land from the ultraviolet radiation from the Sun. 9. Identify one mineral with a red-brown streak that formed when oxygen in Earth’s early atmosphere combined with iron. [1] 10. Identify the temperature zone of the atmosphere in which the ozone layer developed. [1] 11. Complete the pie graph below to show the percent by volume of nitrogen and oxygen gases currently found in Earth’s troposphere. Label each section of the graph with the name of the gas. The percentage of other gases is shown. [1] 8. State one reason why the first rocks on Earth were most likely igneous in origin. [1] Base your answers to questions 12 through 15 on the passage below. Asbestos Asbestos is a general name given to the fibrous varieties of six naturally occurring minerals used in commercial products. Most asbestos minerals are no longer mined due to the discovery during the 1970s that long-term exposure to high concentrations of their long, stiff fibers leads to health problems. Workers who produce or handle asbestos products are most at risk, since inhaling high concentrations of airborne fibers allows the asbestos particles to become trapped in the workers’ lungs. Chrysotile is a variety of asbestos that is still mined because it has short, soft, flexible fibers that do not pose the same health threat. 12. State one reason for the decline in global asbestos use after 1980. [1] 13. Chrysotile is found with other minerals in New York State mines located near 44° 30' N, 74° W. In which New York State landscape region are these mines located? [1] 14. What determines the physical properties of minerals, such as the long, stiff fibers of some varieties of asbestos? [1] 15. The chemical formula for chrysotile is Mg3Si2O5(OH)4. State the name of the mineral found on the Earth Science Reference Tables that is most similar in chemical composition. [1] Base your answers to questions 16 and 17 on the map below. The map shows the approximate area in a portion of North America where some sedimentary rock layers composed of gypsum, halite, and potassium salt minerals are found in Earth’s crust. 16. Identify one New York State landscape region in which deposits of gypsum and halite are commonly found. [1] 17. Identify the sedimentary rock composed of halite and explain how this rock is usually formed. [1] Base your answers to questions 18 and 19 on the diagram below of a mineral classification scheme that shows the properties of certain minerals. Letters A through G represent mineral property zones. Zone E represents the presence of all three properties. For example, a mineral that is harder than glass, has a metallic luster, but does not have cleavage, would be placed in zone B. Assume that glass has a hardness of 5.5. 18. In which zone would the mineral potassium feldspar be placed? [1] 19. State the name of one mineral listed on the Properties of Common Minerals Table that could not be placed in any of the zones. [1] Graywacke Graywacke is a type of sandstone composed of a great variety of minerals. Unlike a “clean” sandstone where both the sandsized grains and cement are composed mostly of quartz, graywacke is a “dirty” sandstone which can be composed of potassium feldspar, plagioclase feldspar, calcite, hornblende, and augite, as well as quartz. Graywacke can be used for paving highways. The hard, massive bedrock is first drilled and then blasted into large chunks. Stone crushers grind these chunks into pebble-sized pieces. Truckloads of the graywacke pebbles are then hauled to plants where asphalt for paving is made. Base your answers to questions 20 through 22 on the passage below. 20. State one difference in the mineral composition of a “clean” sandstone and a “dirty” sandstone. [1] 21. Identify one rock-forming process that must have occurred after the sediments were deposited to form graywacke. [1] 22. State one negative environmental impact a graywacke quarry could have on the area where it is located. [1]