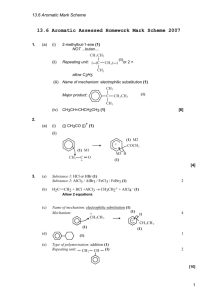
Benzene

L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
CH
3
Chpt. 32: p.1
Benzene and other Arenes
(A) Introduction
Benzene, C
6
H
6 is the simplest member of the class of hydrocarbon called aromatic hydrocarbons or arenes. It is a colourless liquid with a characteristic smell.
These aromatic compounds contain a ring of sp
2 carbon atoms, with a delocalized system of
electrons.
Aromatic compounds (including aromatic hydrocarbons) are stabilized by the
electron delocalization.
The delocalized formulae for benzene and other arenes are shown below:
CH
3
CH
3
__________________
___________________
NO
2
___________________
Cl
___________________
NH
2
___________________
SO
3
H
___________________
OH
___________________
NO
2
___________________
NO
2
___________________
___________________
NO
2
NO
2
___________________
(B) Reactions of Benzene: General consideration
The benzene ring is a planar hexagon with a cloud of delocalized
electrons lying above and below the ring. The reactions of benzene could involve the attack of an electrophile on the cloud of
electrons and undergo addition reaction.
However, what is found is quite contrary to the expectation. The following table compares some reactions of cyclohexane, cyclohexene and benzene.
Reagent
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
Table Some Reactions of Cyctohexane, Cyclohexene and Benzene
Cyclohexane Cydohexene Benzene
Chpt. 32: p.2
Br
2
/ CCl
4
(in dark)
KMnO
4
/H
+
Conc. H
2
SO
4
H
2
/ finely divided Ni
No reaction Br
2 decolorised readily, no HBr evolved,
No reaction MnO
4
decolorised readily.
No reaction with Br
2 alone, in the presence of Fe filings, Br
2 decolorised slowly and HBr fumes evolved.
No reaction
No reaction H
2
SO
4 absorbed readily. Slow substitution reaction when heated, yellow oil formed.
No reaction One mole absorbs one mole of H
2
One mole absorbs 3 moles at room temperature of H
2
slowly at 150
0
C
Interpretation:
Benzene is LESS reactive than cyclohexene (an alkene) though it contains it electrons as alkenes do.
Reason :
The delocalization of it electrons in the benzene ring leads to considerable aromatic stabilization. Larger amount of energy is required to activate the ring before any reaction could occur.
As a result, the benzene ring requires higher temperature and possibly catalyst before it can react due to the resonance stabilization.
(C) Electrophilic Aromatic Substitution of Benzene
Unlike alkenes. benzene undergoes electrophilic substitution rather than addition.
Examples:
H
Cl
Cl
2
/ AlCl
3
+ H Cl
H
SO
3
H conc. H
2
SO
4
+
H
2
O
The substitution reactions can preserve the aromatic stabilization after electrophilic attack.
3.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
The general reaction of electrophilic aromatic substitution can be represented as follow:
1. Formation of the reactive electrophile E
+
.
2.
Chpt. 32: p.3
Attack by E
+
on the
cloud of the benzene ring. The formed carbocation intermediate involves a high activation energy, therefore this becomes the rate determining step.
+ E+
Formation of the final product by loss of the ring proton from the position of electrophilic attack.
The aromaticity is restored to the ring.
The energy profile for this electrophilic aromatic substitution is shown below :
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
Chpt. 32: p.4
(D) Nitration of Benzene
The substitution of a —H atom by a —NO
2 group is called nitration.
To obtain nitrobenzene. C
6
H
5
NO
2
. benzene is refluxed on a water bath at 60°C with a ‘nitrating mixture” — a mixture of concentrated nitric acid and sulphuric acid. nitrobenzene is a pale yellow liquid which can be separated from benzene by distillation under reduce pressure.
Mechanism:
1. The generation of the nitronium ion NO
2
+
Sulphuric acid serves as a strong acid and donate a proton to nitric acid which acts as a base.
2. The nitronium ion NO
2
+ adds to the benzene ring to form the short-lived intermediate, in which both the entering -NO
2
group and the leaving —H atom are bonded to the ring.
3. The intermediate rapidly loses a proton, restoring the symmetry and stability of the benzene ring. The proton is immediately picked up by a hydrogen sulphate ion.
Note <1> The 2nd step is the rate determining step.
Reason:
<2> If the temperature is raised to 95°C, and the fuming nitric acid is used.
1,3—dinitrobenzene is formed.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
Chpt. 32: p.5
(E) Halogenation of benzene
The addition of halogens to the benzene ring takes place under conditions which favour the formation of free radicals, i.e., sunlight or high temperature. The first step in the addition is the homolysis of the halogen molecule, e.g.
Cl
2
2Cl
A different type of reaction takes place at room temperature in the presence of a Friedel—Crafts catalyst Substitution then occurs. e.g.
1
2.
Mechanism of halogenation
Reaction : Benzene reacts with bromine to form bromobenzene (with the presence of FeBr
3
)
The
electron cloud interacts with the bromine molecule, the Br—Br bond becomes polarised
The friedel—Crafts catalyst accepts a pair of electrons from the 6—Br atom in the polarised Br
2 molecule, and enables the Br—Br bond to split. A bromonium ion and a FeBr
4
complex ion are formed.
Br Br FeBr
3
3. The bromonium ion rapidly loses a proton to form bromobenzene, with the generation of the catalyst, FeBr
3
.
Note: lodination of benzene does not occur but some benzene derivatives, containing activating substituents, are iodinated.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
(F) Sulphonation of Benzene
Chpt. 32: p.6
Sulphonation is the substitution of a —H atom by an —SO
3
H group. Benzenesulphonic acid,
C
6
H
5
SO
3
H. is obtained by refluxing benzene with concentrated sulphuric acid for many hours. or warming with fuming sulphuric acid (which contain SO
3
) at 40
0
C for 20-30 minutes.
H reflux with conc. H
2
SO
4
+ H
2
SO
4
Mechanism.
1. Sulphur trioxide is produced in the following equilibrium in which sulphuric acid acts as both an acid and a base.
2H
2
SO
4
SO
3
+ H
3
O
+
+ HSO
4
-
2. Sulphur trioxide is a powerful electrophile and react with the aromatic n system to form a neutral but dipolar intermediate.
O
S O
O
3. The intermediate is attacked by the hydrogen sulphate ion HSO
4
-
to form the product.
Note : <1> Unlike nitration, halogenation, etc. sulphonation is reversible. By heating an
0 aqueous solution of benzenesulphonic acid above 100
0
C, benzene and sulphuric acid are formed.
So, the choice of conditions determines the direction of the reaction.
<2> The —SO
3
H group in the product can be replaced by hydrolysis to give an —OH group.
Benzenesulphonic acid is converted to phenol. C
6
H
5
OH. This is the easiest way of preparing phenol in the laboratory.
<3> Sulphonation is important in the manufacture of detergents because detergents contain —SO
3
H groups that they lather even in hard water, as the calcium and magnesium salts of sulphonic acid are soluble.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
Chpt. 32: p.7
(G) Alkylation of benzene
An alkyl group can be introduced into the benzene ring by the reaction of a halogenoalkane with benzene.
Such type of reaction is called Friedel-Crafts reaction. The reaction takes place under the influence of a catalyst, such as aluminium chloride (AlCl
3
) , aluminium bromide (AlBr
3
) and iron(III) bromide (FeBr
3
)
Such catalysts function as Lewis acid since the central atom can accept a pair of electrons. e.g.
AlC1
3 can accept a pair of electrons from a chloride ion.
AlCl
3
+ Cl
AlCl
4
-
Mechanism of alkylation
When a Friedel—Crafts catalyst (e.g. A1Br
3
) is dissolved in a halogenoalkane,
1. A1Br
3 can accept the bromide ion from C
2
H
5
Br, forming a carbocation, C
2
H
5
+
.
C
2
H
5
Br + AlBr
3
C
2
H
5
+
+ AlBr
4
-+
2. The attack on the it electron cloud of the benzene ring by the C
C
2
H
5
+
A1Br
4
-
, forming an intermediate.
2
H
5
+
or by the complex
C
2
H
5
+
3. The reactive intermediate quickly loses a proton to form the product, ethylbenzene. The catalyst,
AlBr
3
is regenerated, and hydrogen bromide is evolved:
Note:
Alkylation can also be affected by the use of an alkene and a Friedel—Crafts catalyst together with and acid such as HCl or H
3
PO
4
:
The first step in the reaction is protonation of the alkene by the acid to form a carbocation which attacks the benzene ring.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 32: Aromatic Hydrocarbon
(H) Methylbenzene (Toluene)
CH
3
Chpt. 32: p.8
Methylbenzene (or toluene) C
6
H
5
CH
3
. resembles benzene. Its physical properties are similar : the boiling tegiperature is higher (111
0
C) and the melting temperature lower (-95
0
C)
Reactions of the ring : Electrophilic substitution
Methylbenzene is more reactive than benzene towards the electrophilic reagents which substitute in the ring.
Reason : The methyl group can pushes electrons into the ring through positive inductive effect .
Milder conditions are employed than in the reactions of benzene.
<1> Suiphonation : fuming H
2
SO
4
(containing SO
3
)
<2> Nitration : Conc. HNO
3
+ conc. H
2
SO
4 at 30°C
A mixture of 1,2— and 1.4—substituted ,rnethylbenzenes obtained in each case.
CH
3
H
+ NO
2
+
If the temperature is raised, two groups or three groups are introduced.
CH
3
CH
3
CH
3
NO
2
O
2
N
NO
2
NO
2
NO
2
NO
2