October 2009 Quiz with answers
advertisement
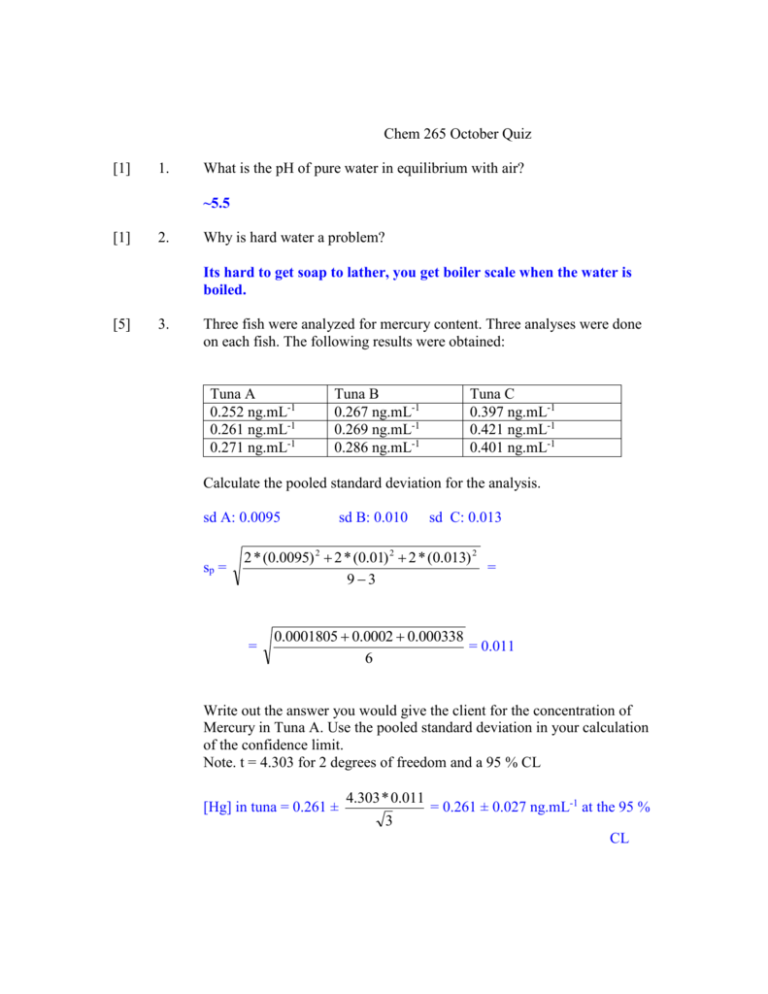
Chem 265 October Quiz [1] 1. What is the pH of pure water in equilibrium with air? ~5.5 [1] 2. Why is hard water a problem? Its hard to get soap to lather, you get boiler scale when the water is boiled. [5] 3. Three fish were analyzed for mercury content. Three analyses were done on each fish. The following results were obtained: Tuna A 0.252 ng.mL-1 0.261 ng.mL-1 0.271 ng.mL-1 Tuna B 0.267 ng.mL-1 0.269 ng.mL-1 0.286 ng.mL-1 Tuna C 0.397 ng.mL-1 0.421 ng.mL-1 0.401 ng.mL-1 Calculate the pooled standard deviation for the analysis. sd A: 0.0095 sp = sd B: 0.010 sd C: 0.013 2 * (0.0095) 2 2 * (0.01) 2 2 * (0.013) 2 = 93 = 0.0001805 0.0002 0.000338 = 0.011 6 Write out the answer you would give the client for the concentration of Mercury in Tuna A. Use the pooled standard deviation in your calculation of the confidence limit. Note. t = 4.303 for 2 degrees of freedom and a 95 % CL [Hg] in tuna = 0.261 ± 4.303 * 0.011 3 = 0.261 ± 0.027 ng.mL-1 at the 95 % CL [4] A sample contained clay particles and iron ore particles. The clay particles made up 0.20 of the sample. For a sample containing 1.00 x 106 particles, what is the relative sampling standard deviation for the clay particles. sd = npq 1.00 x10 6 * 0.20 * 0.80 1.6 x10 5 400 Rel. sd for clay = 400 0.002 or 0.2% 1x10 6 x0.2 How could you reduce the sampling error? By grinding the sample or taking a larger amount. [2] What acid (hint – it’s a mix) is used to dissolve gold? Give the name and composition. Aqua regia – nitric acid and hydrochloric acid [1] What do you add to a sample to volatilize silicates? HF [2] What do we mean when we say we would like to know the ‘speciation of arsenic’ in a pond. It means we would like to know the concentration of each type of arsenic ion and arsenic complex. (Different forms show different toxicities) [1] Why do you have to be especially careful when using perchloric acid? (As opposed to the normal precautions you would take for using any other acid like HCl). Perchoric acid reacts with organics to form perchlates species which can be explosive when dry. [2] Would you add acid or base to a sample containing metals if you wanted to make sure they stayed in solution? Explain why. Acid – metals form insoluble hydroxides in base. [2] Write an equation for the reaction which took place when someone carrying a bottle of hydrochoric acid spilled it on the marble floor in the PSC building. 2H CO 32- H 2 CO3 H 2 O CO2 [2] What happens to a sodium hydroxide solution when you leave it stored in a ‘plastic’ bottle or sitting open on a bench? Carbon dioxide dissolves in it and forms carbonates Write the equation H 2 O CO2 H 2 CO3 H 2 CO3 2OH 2 H 2 O CO3 [2] Distinguish between trace and micro analysis. Trace: there is a low concentration in the sample Micro: you have a very small sample size [1] State Beers Law (give formula) A = Єbc [2] A 3.96 x 10-4 M solution of neocuproine exhibited an absorbance of 0.624 at 454 nm in a 1.00 cm cuvette. A blank solution had an absorbance of 0.029 at the same wavelength. Find the molar absorptivity of neocuproine. 0.624 0.029 = 1502 L.M-1.cm-1 1cm * 3.96 x10 4 So 1.50 x 103 L.mol-1.cm-1 Є = A/bc = [2] Draw a block diagram for an absorption spectrometer. Light - Wavelength selector - sample cell - detector [4] Find the equilibrium concentration of Pb2+ in a solution containing 0.10 M bromide ion and with solid PbBr2(s) in the flask. The Ksp for PbBr2 = 2.1 x 10-6 PbBr2(s) ↔ Pb2+ + 2 BrKsp = [Pb2+] [Br-]2 Let x = the concentration of Pb2+ Then [Br-] = 2x + 0.10 M Assume x<0.10 Ksp = x * (0.10)2 = 2.1 x 10-6 So x = 0.00021 M = [Pb2+] Check assumption: 2.1 x 10-4 < 2.1 x 10-6 Difference is 100 fold – so OK. [4] 7. Write the Ka and Kb reactions and equilibrium constant expressions for NaHCO3. HCO3- ↔ CO3- + H+ [CO3 ][ H ] Ka = HCO3 HCO3 + H2O ↔ H2CO3 + OH - - Kb = [ H 2 CO3 ][OH ] [ HCO3 ]