Supplementary Figures (doc 3141K)
advertisement

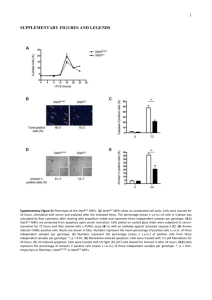
Supplementary Information. Dewson et al. Figure S1. Bax activation can be monitored by disulphide-linkage of cysteines substituted at Bak-equivalent positions. bax-/-bak-/- MEFs expressing the double-cysteine Bax variant, S4C/L148C, were treated or not with etoposide. Membrane and cytosol were separated and disulphide bonds induced with oxidant as in Figure 1b. Data is representative of two independent experiments. 1 Figure S2. Specificity of BH3:groove linkage in oligomerized Bax. Membranes from MEFs expressing pairs of FLAG- and HA-tagged single-cysteine variants of Bax S184L were treated with tBid as in Figure 2d. Disulphide-linkage was induced by CuPhe and the samples immunoprecipitated for FLAG prior to non-reducing or reducing SDS-PAGE. Immunoprecipitates (IP) and cell lysates were western blotted (WB) for HA or FLAG, as indicated. Data is representative of two independent experiments. 2 Figure S3. Orientation of the BH3 domain in the hydrophobic groove. (a) Model of the BH3:groove interaction in Bax homodimers. A model of a BH3 domain (based on Bim; red) bound to the Bax hydrophobic groove (3 and 4; aa74-100; green) is as shown in Figure 2a. Residues mutated to cysteine are indicated. (b) The Bax E69C mutant retains apoptotic function. bax-/-bak-/- MEFs expressing FLAG-tagged Bax E69C were treated with etoposide (10 M). Cell death is expressed as mean ± SD of three independent experiments. (c) E69C in the BH3 domain fails to link to two groove cysteines. MEFs expressing pairs of FLAGand HA-tagged single-cysteine Bax variants were treated with etoposide (10 M). Cytosolic (C) and mitochondrial (M) fractions were incubated with CuPhe and immunoprecipitated for FLAG prior to non-reducing or reducing SDS-PAGE. Immunoprecipitates (IP) and cell lysates were western blotted (WB) for HA or FLAG, as indicated. Data is representative of two independent experiments. Note the low efficiency of disulphide-linkage of E69C to R94C or V95C (lanes 8 and 12 respectively) compared with linkage of S55C to R94C (lane 4). Note also that Bax dimers were detectable only in the membrane fraction of etoposide-treated cells. 3 Figure S4. The Bax N-segment is not required for Bax activation, oligomerization or function. (a) Bax lacking its N-terminal 5 or 10 amino acids retains apoptotic function. bax-/-bak-/- MEFs expressing the indicated Bax variants were treated with etoposide (10 M). Cell death is expressed as mean ± SD of three independent experiments. Bax expression levels in untreated cells were assessed by immunoblotting for Bax, and for HSP70 as a loading control. (b) Truncated Bax still oligomerizes after etoposide treatment. MEFs expressing the indicated Bax variants were treated with or without etoposide, and cytosol (C) and membrane (M) fractions analyzed by BN-PAGE and immunoblotted for Bax. Fractions were also run on reducing SDS-PAGE and immunoblotted for cytochrome c, HSP70 or VDAC1. Data is representative of two independent experiments. (c) Bax G10C exposes the 6A7 epitope following etoposide treatment. MEFs expressing Bax or Bax G10C were left untreated or treated with etoposide (10 M) prior to lysis in CHAPS and immunoprecipitation with the conformation-specific 6A7 antibody. Data is representative of three independent experiments. 4 Figure S5. An 66 interface can link symmetric BH3:groove dimers in Bax oligomers. (a) Disulphide-linked Bax oligomers convert to monomers under reducing conditions. Samples shown in Figure 5c were run under reducing conditions. (b) BH3:groove dimers can link via an6:6 interface to trap higher order complexes. bax-/-bak-/MEFs expressing the indicated single-, double-, or triple-cysteine Bax variants were left untreated or treated with etoposide (10 M). Membrane fractions were isolated, treated with CuPhe and analyzed by non-reducing SDS-PAGE. Note the different migration of dimers linked via the BH3:groove and 6:6 interfaces. 5