Word file (34 KB )
advertisement

SUPPLEMENTAL DATA (Tables)
Table S1: Analysis of Humanin Mutants: Correlation with Bax-binding and antiapoptotic function.
HN Peptide
Bax binding
Bax suppression
1 – 24
+
+
+
+
+
+
+
+
-
+
+
+
+
+
+
+
+
-
3 – 24
4 – 24
7 – 24
10 – 24
13 – 24
1 – 17
1 – 15
1 – 12
3 – 19
3 – 18
3 – 17
C8P
L9R
GFP Only
Wild-type Humanin peptide (1 – 24) or various truncated or site-specific mutant peptides
were expressed as GFP fusions in HEK293 cells by transient transfection. Peptides
were tested for binding to Bax by co-immunoprecipitation assay using lysates from
transfected cells (“Bax-binding”). Peptide-mediated suppression of apoptosis induced
by transfection of Bax-encoding plasmid was also assayed (“Bax suppression”), scoring
as positive GFP-HN mutants that suppressed Bax-induced apoptosis by 50%.
Immunoblotting confirmed production of all GFP-HN peptides at comparable levels (not
shown). Also, GFP-HN did not affect levels of Bax protein production. Data in Table
represent a summary based on at least two independent determinations.
2
SUPPLEMENTAL DATA (Figures)
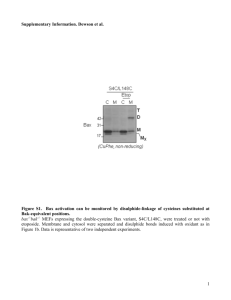
Figure S1. HN is located in cytosol. (A) SF268 cells were fractionated by differential
centrifugation to produce heavy membrane (pellet [P]) and cytosolic (supernatant [S])
fractions, which were analyzed by immunoblotting using anti-HN (top), -Bax (second), Hsp60 (third) [mitochondrial marker], -tubulin (fourth) [cytosol marker], or Grp78 (ERmarker) (bottom), antibodies.
(B) Plasmids encoding GFP fusion proteins with HN
attached at either the C-terminal (N2-GFP) or N-terminal (C1-GFP) end were
transfected into Cos-7 cells. Cells were stained with DAPI and imaged by confocal
microscopy.
Note that GFP-HN fusion proteins suppress Bax-induced apoptosis,
regardless of whether HN is appended at N-terminus or C-terminus of GFP (not shown).
Figure S2. Specific cell death sensitization by Humanin siRNA.
To confirm the
specificity of cell death sensitization induced by Humanin (HN) siRNA, we compared the
effects of transfecting a mixture of two HN siRNAs, HN-siRNA1 + HN-siRNA2 (white
bars) into SF268 cells, with the effects of transfection of an equivalent amount of a
mixture of scrambled-control double-strand RNAs, mut-siRNA1 + mut-siRNA2 (black
bars), and with mock-transfected cells exposed to transfection reagent (oligofectamine)
without nucleic acids (striped bars), and with untransfected cells (cross-hatched bars).
Cells were counted by DAPI assay to determine the percentage of apoptotic cells,
evaluating 200 GFP-positive cells per sample (mean SD; n = 3). Note that mocktransfection and that transfection of control dsRNAs did not increase the sensitivity of
SF268 cells to apoptosis induced by STS.
3
Figure S3. Humanin mutant (L9R) does not interact with Bax and does not rescue
cells from Bax-induced apoptosis.
The L9R mutant of Humanin was previously
reported to lack anti-apoptotic activity 4, prompting us to test its ability to bind Bax. (A)
HEK293T cells were transfected with pcDNA3-HA-Bax together with plasmids encoding
GFP, GFP-HN, or GFP-HN(L9R). Cell lysates were immunoprecipitated with polyclonal
anti-GFP antibody.
The immunoprecipitates (IP) or cell lysates were analyzed by
immunoblotting using anti-HA or anti-GFP antibodies, respectively. (B) pcDNA3-HA-Bax
plasmids were co-transfected with GFP, GFP-HN, or GFP-HN(L9R) plasmids into
CSM14.1 cells. Apoptotic cells were counted by DAPI assay 48 h after transfection.
Figure S4. Humanin peptide prevents STS-induced Bax translocation in intact
cells. Cos-7 cells were first transfected with GFP-Bax plasmids. After 24 hrs, untagged
wild-type or C8P mutant HN peptides were introduced into cells using Chariot TM
reagent, and 2 h later cells were treated with 1 M STS. After 4 h, cells were imaged by
confocal microscopy. Representative photomicrographs are shown.
Figure S5. HN peptide stabilizes conformations of Bax that bind the C-terminal
transmembrane domain. Solution NMR and antibody-based epitope mapping studies
suggest that the mechanism of conversion of Bax from latent to active form involves the
dislodging of a C-terminal hydrophobic -helix (transmembrane [TM] domain) from the
body of the Bax protein, exposing this membrane-anchoring TM domain for insertion
into mitochondrial membranes 8. To test the effects of HN on the interaction of the TM
domain of Bax with the rest of the Bax protein, we separately produced a C-terminally
4
truncated, Flag-tagged Bax protein (residues 1-169) (Flag-BaxTM) and a GFP-fusion
containing the C-terminal TM domain of Bax (residues 170-192) by expression in
HEK293T cells. Lysates from the independently transfected cells were then used as a
source of Flag-BaxTM and GFP-HM proteins, respectively. Lysates containing the
Flag-BaxTM protein were pre-incubated with HN or control peptide (HN C8P), then
mixed with GFP-TM, testing for interaction by co-immunoprecipitation assay using antiFlag antibody to immunoprecipitate Bax(TM) and anti-GFP antibody for detecting
associated GFP-TM by immunoblotting. In the absence of HN, no binding of GFP-TM
to BaxTM was detected. In contrast, when HN was added, GFP-TM was coimmunoprecipitated with BaxTM. HN (C8P) peptide did not promote interaction of
GFP-TM with BaxTM, demonstrating the specificity of these results.
Thus, HN
appears to stabilize the latent conformation of Bax (previously delineated by solution
NMR), in which its C-terminal tail is docked onto a hydrophobic crevice on the surface of
the Bax molecule. Normally, this is an intra-molecular interaction, but in the assay
designed here, we have used fragments of Bax (Bax TM versus TM) to create an
assay based on inter-molecular interaction. We speculate therefore that HN does not
physically link the TM domain to the body of the Bax protein, but rather that HN interacts
with sites on Bax that stabilize its TM-binding, latent conformation.
Figure S6.
Humanin homologs in other species.
The predicted amino-acid
sequences are presented in single-letter code for HN-related peptides of various
species. The sequences shown, represent virtual translations of ORFs found in the
following cDNAs: BQ250660 {Wheat Triticum aestivun}; AI209224 {Nematode
Onchorcerca volvulus}; and BG667570 {Rat}.
5
SUPPLEMENTAL INFORMATION
Additional details of methods and procedures are provided here.
Yeast two-hybrid screen: One of the positive clones demonstrating interactions with
Bax (S184K) (expressed as a fusion protein with the DNA-binding domain of LexA) was
determined to contain a HN cDNA, cloned in-frame with the upstream B42 Transactivation Domain (TAD). The interaction between HN and Bax was further confirmed
by re-transformation of plasmids into EGY48 yeast cells. Co-expressing pGilda-Bax
(S184K) and pYESTrp-Humanin in EGY48 cells harboring a lexA-LEU2 reporter gene
allowed for their growth on leucine-deficient media, while cells containing either Bax
(S184K) or Humanin alone did not. Various control plasmids also failed to support
growth on leucine-deficient media when combined with pGilda-Bax(S184K) or pYESTrpHumanin.
Plasmid Constructions. A cDNA containing the ORF of Humanin without additional
flanking sequences was generated by PCR using an EST clone encoding full-length HN
as a template (BE899497). The resulting PCR products were digested with restriction
endonucleases and subcloned into the Xho I and Hind III sites of pEGFP-C1 and Xho I
and EcoR I sites of pEGFP-N2 (Clontech). Truncation and site-specific mutants of
Humanin were created by PCR.
Apoptosis assays.
Both floating and adherent cells (after trypsinization) were
collected 48 hr after transfection, fixed, and stained using 4’,6-diamidine-2’-phenylindole
6
dihydrochloride (DAPI) for assessing apoptosis based on nuclear fragmentation and
chromatin condensation.
Peptides. Rhodamine-conjugated HN peptide was synthesized on MBHA resin and
amidated at the C-terminus. 1-aminohexanoic acid(ahx)-Humanin was initially prepared
with an Advanced Chemtech 350 multiple peptide synthesizer using standard
fluorenylmethoxycarbonyl chemistry with DIC coupling.
Rhodamine B (Aldrich) was
coupled to ahx-Humanin using N-[(Dimethylamino0-1H-1,2,3-triazolo[4,5-b]pyridin-1ylmethylene]-N-methylmethanaminium
hexafluorophosphate
N-oxide,
1-
hydroxybenzotriazole hydrate, and Diisopropylethylamine and occasional sonication
until the ninhydrin test was negative. The peptide was deprotected and cleaved from the
resin, precipitated with ice-cold diethyl ether and purified by HPLC on a reverse-phase
C18 Cosmosil column, eluted with a water-acetonitile, 0.1% trifluoroacetic acid gradient
and analyzed by matrix-assisted laser desorption/ionization (MALDI)- time-of-flight
(TOF) mass spectrometry.
7