Lecture:
advertisement
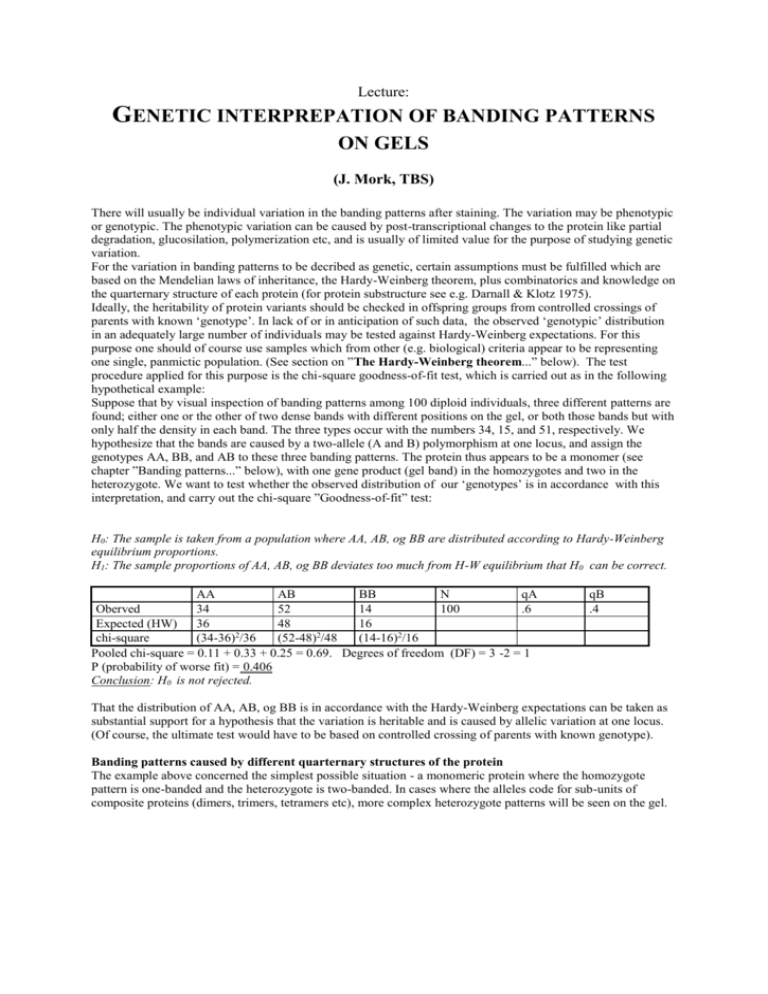
Lecture: GENETIC INTERPREPATION OF BANDING PATTERNS ON GELS (J. Mork, TBS) There will usually be individual variation in the banding patterns after staining. The variation may be phenotypic or genotypic. The phenotypic variation can be caused by post-transcriptional changes to the protein like partial degradation, glucosilation, polymerization etc, and is usually of limited value for the purpose of studying genetic variation. For the variation in banding patterns to be decribed as genetic, certain assumptions must be fulfilled which are based on the Mendelian laws of inheritance, the Hardy-Weinberg theorem, plus combinatorics and knowledge on the quarternary structure of each protein (for protein substructure see e.g. Darnall & Klotz 1975). Ideally, the heritability of protein variants should be checked in offspring groups from controlled crossings of parents with known ‘genotype’. In lack of or in anticipation of such data, the observed ‘genotypic’ distribution in an adequately large number of individuals may be tested against Hardy-Weinberg expectations. For this purpose one should of course use samples which from other (e.g. biological) criteria appear to be representing one single, panmictic population. (See section on ”The Hardy-Weinberg theorem...” below). The test procedure applied for this purpose is the chi-square goodness-of-fit test, which is carried out as in the following hypothetical example: Suppose that by visual inspection of banding patterns among 100 diploid individuals, three different patterns are found; either one or the other of two dense bands with different positions on the gel, or both those bands but with only half the density in each band. The three types occur with the numbers 34, 15, and 51, respectively. We hypothesize that the bands are caused by a two-allele (A and B) polymorphism at one locus, and assign the genotypes AA, BB, and AB to these three banding patterns. The protein thus appears to be a monomer (see chapter ”Banding patterns...” below), with one gene product (gel band) in the homozygotes and two in the heterozygote. We want to test whether the observed distribution of our ‘genotypes’ is in accordance with this interpretation, and carry out the chi-square ”Goodness-of-fit” test: H0: The sample is taken from a population where AA, AB, og BB are distributed according to Hardy-Weinberg equilibrium proportions. H1: The sample proportions of AA, AB, og BB deviates too much from H-W equilibrium that H0 can be correct. AA AB BB N qA Oberved 34 52 14 100 .6 Expected (HW) 36 48 16 chi-square (34-36)2/36 (52-48)2/48 (14-16)2/16 Pooled chi-square = 0.11 + 0.33 + 0.25 = 0.69. Degrees of freedom (DF) = 3 -2 = 1 P (probability of worse fit) = 0.406 Conclusion: H0 is not rejected. qB .4 That the distribution of AA, AB, og BB is in accordance with the Hardy-Weinberg expectations can be taken as substantial support for a hypothesis that the variation is heritable and is caused by allelic variation at one locus. (Of course, the ultimate test would have to be based on controlled crossing of parents with known genotype). Banding patterns caused by different quarternary structures of the protein The example above concerned the simplest possible situation - a monomeric protein where the homozygote pattern is one-banded and the heterozygote is two-banded. In cases where the alleles code for sub-units of composite proteins (dimers, trimers, tetramers etc), more complex heterozygote patterns will be seen on the gel. Dimeric proteins: Three combinations of sub-units X and Y are possible (XX, XY, and YY). The heterozygote will show one Xband, one intermediate XY-band, and one Y-band. The relative amounts (and staining intensity) of the three types will, according to simple combinatorics, be 1:2:1. Trimeric proteins: Four possible combinations: XXX, XXY, XYY, YYY with expected intensity 1:3:3:1. Tetrameric proteins: Five possible combinations: XXXX, XXXY, XXYY, XYYY, YYYY with expected intensities 1:4:6:4:1. Interlocus hybrid bands When several loci code for sub-units of composite proteins one will often (but not always) find molecules which are composed of sub-units from different loci. In the simplest case; two monomorphic loci for a dimeric protein, this may be manifested as one hybrid zone in the middle between the two homodimeric bands of the two loci. The banding pattern will be substantially more complex when looking at multiple loci coding for sub-units of tetrametic proteins. In general I, the theoretically expected number of bands will be (Harris & Hopkinson 1977): I = (L + h + n -1)! / n!(L + h -1)! Where L=number of loci, h=number of heterozygous loci per individual, and n=number of sub-units per protein. For example, a double heterozygote for LDH-2* og LDH-3* in cod will be expected to show: I = [(2+2+4-1)!/ (4!(2+2-1)!)] = 35 bands. For a triple heterozygote the number would be 126. Inter-specific hybrid patterns Species hybrids usually show the protein bands from both parent species. Since even closely related species rarely have the same alleles at a locus, electrophoresis is a very efficient method both for the identification of species and the detecting species hybrids. Particularly, this applies to the younger stages in species where species characteristics develop at more advanced stages of development, and for species which in general are difficult to identify morphologically. The method has, e.g., been applied with success on eggs and adults of salmon/trout and various mosses and their hybrids. Patterns in lower and higher levels of ploidy In haploid species, only one allele is manifested and therefore only one band is seen at each locus. The existence of ‘heterozygous’ banding patterns in such species must therefore be due to inter-locus hybrid bands. Some species (e.g. salmon, several grass species etc) are natural popyloids and may have double, triple etc sets of genes at their loci. Polymorphisms in such species may show patterns and staining intensities which deviate from those shown in the figure above, mainly with respect to the symmetry in band intensities of the heterozygotes. The reason for this is that proteins synthesized at several loci may have the same electrophoretic mobility (lying ‘on top’ of each other in the gel). If, for example, an individual is heterozygous for a dimeric protein on a duplicated locus, one of the homodimers will be more intensively staining than the other, because the homodimers from the other (monomorphic) locus have the same electrophoretic mobility. Testing the zymograms with gel-scanners has shown good correlation between observed and expected staining intensity in cases where the individuals were scored as heterozygotes on a duplicated locus. Thus in practice the genotype scoring needs not be a problem at higher levels of ploidy. References for chapter «Protein electrophoresis» Darnall, D. W. & Klotz, I.M. 1975. Subunit constitution of proteins - A Table. Arch. Biochem. Biophys. 166: 651- 682. Harris, H. & Hopkinson, D.A. 1977. Handbook of enzyme electrophoresis in human genetics. Elsevier/Noth Holland Biomedical Press. Westermeier, R. 1993. Electrophoresis in practice. VCH Publishers Inc., NY. 277 pp. ISBN 1-56081-705-4. ooooooooooooooOOOOOOOOOOOOooooooooooooooooo







