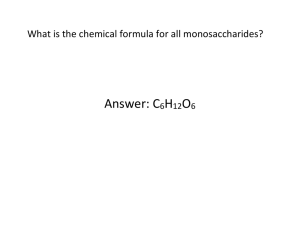
DIGESTION OF CARBOHYDRATES
advertisement

DIGESTION OF CARBOHYDRATES The principal sites of dietary carbohydrate digestion are the mouth and intestinal lumen . This digestion is rapid and is generally completed by the time the stomach contents reach the junction of the duodenum and jejunum . There is little monosaccharides present in deits of mi8xed animal and plant origin ; thus , the enzymes needed for degradation of most dietary carbohydrates are primarily disaccharides and endoglycoidases ( for breaking down oligosaccharides and polysaccharides ) . Hydrolysis of glycosidases that degrade carbohydrates into their reducing sugar components . These enzymes are usually specific for the structure and configuration of the glycosyl to be removed as well as the type of bond to be broken . A. Digestion of carbohydrates begins in the mouth The major dietary polysaccharides can be of animal ( glycogen ) or plant origin ( starch , composed of amylose and amylopectin ) . During mastication , salivary α- amylase ( ptyalin ) acts briefly on dietary starch in random manner , breking some α- ( 1-4 ) bonds . [Note : carbohydrates are the only dietary component for which degradation begins in the mouth ] 1. There are both α- and β- ( 1-4 ) – endoglucosidases in nature , but humans do not produced and secrete the latter in digestive juices . Therefore , they are unable to digest cellulose , a carbohydrate of plant origin containing β- ( 1-4 ) glycosidic bonds between glucose residues 2. Because amylopectin and glycogen also contain α- ( 1-6 ) bonds , the digest resulting from the action of α- amyloase contains a mixture of smaller , branched oligosaccharides molecules . 3. Carbohydrates digestion halts temporarily in the stomach , because the high acidity inactivates the salivary a–amylase . B- Further digestion of carbohydrates by pancreatic enzymes Occurs in the small contents . When the acidic stomach contents reach the small intestine , they are neutralized by bicarbonate secreted by the pancreas , and pancreatic α-amylase continues the process of starch digestion . C. Final carbohydrate digestion by enzymes by the intestinal mucosal cells . The final digestion processes occur at the mucosal lining of the upper jejunum , declining as they proceed down the small intestine , and include the action of several disaccharides and oligosaccharides . These 1 enzymes are secreted through , and remain associated with the luminal side of the brush border membranes of the intestinal mucosal cells . Absorption of monosaccharides by intestinal mucosal cells The duodenum and upper jejunm absorb the bulk of the dietary sugars . Insulin is not required for the p ** of glucose by intestinal cells . However different sugars have different mechanisms of absorption . Two mechanisms are responsible for the absorption of monosaccharides : Active transport against concentration gradient , and passive diffusion . 1F Active transport : galactose and glucose are transported into the mucosal cells by an active , **** requiring that involves a specific transport protein ( carrier ) and requires a concurrent uptake of sodium ions . The carrier binds glucose and sodium at separate sites and transports them through the membrane of the intestinal cell . Both glucose and sodium are released into the cytosol , allowing the carrier to take more “cargo” . In this way , sodium is transported down its concentration gradient and at the same time causes the carrier to transport glucose against its concentration gradient . The free energy required for the active transport is obtained from the hydrolysis of ATP linked to a sodium pump that expels sodium from the cell in exchange for k+ 2] Simple diffusion : fructose and glucose also are transported by facilitated glucose transporters by diffusion according to concentration gradient . Abnormal degradation of disaccharides The overall process of carbohydrate digestion and absorption is so efficient in healthy individuals that ordinarily all digestible dietary carbohydrate is absorbed by the time the ingested material reaches the lower jejunum . However , because predominantly monosaccharides are absorbed , any defect in a specific disaccharides activity of the intestinal mucosa . As a consequence of the presence of this osmotically active material , water is drawn from the mucosa into the large intestine , causing osmotic diarrhea. This is reforced by the bacterial fermentation of the remaining carbohydrate of two- and three-carbon compounds ( which are also osmotically active ) plus large volumes of Co2 and H2 gas , causing flatulence . 2 1. Lactase intolerance : More than half of the world’s adults are lactose intolerant . This is particularly manifested in certain races : adult blacks and Asians . The mechanism by which the enzyme is lost is not clear , but is determined genetically and represent a reduction in the amount of enzyme protein rather than a modified enzyme protein rather than a modified inactive enzyme . Treatment for this disorder is simply to remove lactose from the diet . 2. Isomaltose-sucrase deficiency : This enzyme deficiency results in **** of ingested sucrose . This disorder is found in about 10% of Greenland’s Eskiros, whereas 2% of North . treatment is to **** dietary sucrose . Diagnosis : Identification of the specific enzyme deficiencies can be obtained by performing oral tolerance tests with the individual disaccharides . Measurment of hydrogen gas in the breath is a suitable test for determining the amount of gested carbohydrate not absorbed by body but rather metabolized by the intestinal flora . Out line of Glucose Metabolism Glucose is the main carbohydrate present in blood . The metabolism of glucose involves many metabolic pathways : 1- Oxidative pathways : After glucose is transported into cells it is phosphorylated to glucose 6-Phospate ( G6-p ) which is metabolized to pyruvate and lactate in all mammalian cells by the pathway of glycolysis . Glucose is a unique substrate because glycolysis can occur in the absence of oxygen (anaerobic) , when the end product is lactate only . However , tissues that can utilize oxygen ( aerobic ) ar able to metabolize pyruvate to acetylCoA, which can enter the citric acid cycle for complete oxidation to CO2 and H2O , with liberation of much free energy as ATP in the process of oxidative phosphorylation . Thus , glucose is a major fuel of many tissues. 2- The pentose phosphate pathway : G6-P is also oxidized in the pentose phosphate pathway . Th is a source of reducing equivalents ( 2H ) used for biosynthesis e . g . of fatty acids , and it is also the source of ribose , which is important for nucleotide and nucleic acid formation . 3-Storage as glycogen : 3 G6-P is converted to its storage polymer , glycogen that occurs particularly in skeletal muscle and liver . 4- Fat biosynthesis : Triose phospate ( from glycolysis ) gives rise to the glycerol moiety of Acyl glycerola ( fat ) . 3- Pyruvate and intermediates of the citric acid cycle provide the carbon skeletons for the synthesis of amino acids , and acetyl – COA in the building block for long-chain fatty acids and cholesterol , the precursor of all steroids synthesized in the body . 4- Gluconeogenesis is the process that produces glucose from non carbohydrate precursors , e . g. . lactate , amino acids , and glycerol . 5- Fructose and galactose in the tissues are converted to intermediates glucose metabolism , thus the fate of these sugars parallels that of glucose . Glycolysis ( Embeden-Meyerhof pathway ) And the Oxidation of pyruvate Over view The glycolytic pathway is employed by all tissues for the break down of glucose to provide energy ( in the form of ATP ) and intermediates for other metabolic pathways . Glycolysis is at thehub of carbohydrate metabolism because virtually all sugars ( whether arising from the diet or from catabolic reactions in the body ) ultimately can be converted to glucose . Pyruvate is the end product of glycolysis in cells with mitochondria and an adequate supply of oxygen . This series of ten reactions is called aerobic glycolysis because oxygen is required to reoxidize the NADH formed during the oxidation of glyceraldehyde 3phospate . Aerobic glycolysis sets the stage for the oxidative decarboxylation of pyruvate to acetyl CoA , a major fuel of the citric acid cycle . Alternatively , glucose can be converted to pyruvate , which is reduced by NADH to form lactate . This conversion of glucose to lactate is called anaerobic glycolysis because there is ni net formation of NADH , and therefore , it can occur in the absence of oxygen . Anaerobic glycolysis allows the continued production of ATP in tissues that lack mitochondria ( e . g . red blood cells ) or in cells deprived of sufficient oxygen ( e . g . skeletal muscles ) . 4 TRANSPORT OF GLUCOSE INTO CELLS The transport of glucose into cells occur by one of two mechanisms : A. Facilitated transport : This is mediated by a family of at least five glucose transporters (GLUT) in the cell membrane , designated GLUT – 1 to GLUT – 5 Extracellular glucose binds to the transport , which then alters its conformation , discharging glucose inside the cell . In facilitated diffusion glucose movement is “with” a concentration gradient , that is , from a high glucose concentration outside the cell to a lower concentration within the cell . B. Cotransport The second mechanism for glucose entry into cells is contransport , an energy-requiring process that transports glucose “against” a concentration gradient , that is , from low glucose concentration outside the cell to higher concentrations within the cell . Cotransport is a carriermediated process in which the movement of glucose is coupled to the concentration gradient of Na+ , which is transported into the cell at the same time . This type of transport occurs in the epithelial cells of the intestine , and renal tubules . REACTIONS OF GLYCOLYSIS All the glycolytic reactions occur in the cytosol . The conversion of glucose to pyruvate ( under aerobic is transferred to lactate . ( Stage IV ) : 1- Stage I : This involves the first three reactions of glycolysis correspond to an energy investment phase where phosporylated forms of glucose and fructose are synthesized at the expense of ATP , which is converted to ADP . 2- Stage II : This involves the splitting of fructose 1,6 bisphosphate in 2 triose phosphates . 3- Stage III : This involves the subsequent reactions of glycolysis that constitute an energy generation phase where a net of two molecules of ATP are formed per glucose molecule metabolized . Two molecules of NADH are formed when pyruvate is produced ( aerobic ) . 4- Stage IV : Under anaerobic conditions , NADH is reconverted to NAD+ , and lactate is the end product . Stage I A] Phosphorylation of glucose : 5 The irreversible phosphorylation reaction effectively traps glucose as glucose 5-phosphate , which does not diffuse out of the cell . Phosphorylated sugar molecules do not readily penetrate cell membranes because there are no specific carriers for these compounds . This commits glucose to further metabolism in the celol . Mammals have several isoenzymes of hexokinase that catalyze the phosphorylation of glucose to glucose 6-phosphate . 1. Hexokinase It is present in most tissues . It has a broad specificity and is able to phosphorylate several hexoses in addition to glucose . It is inhibited by the reaction product , glucose 6-phosphate , that accumulates when further metabolism of this hexose phosphate is reduced , for example , by a high ATP / ADP ratio . Hexokinase has a low Km ( and therefore a high affinity ) for glucose . This permits the efficient phosphorylation and subsequent metabolism of glucose even when tissue concentrations of glucose are low . Hexokinase , however , has a low V max for glucose and therefore cannot phosphorylate large quantities of glucose . 2. Glucokinase : It is present in liver ( and the *-cells of the pancreas ) , it is the predominant enzyme for the phosphorylation of glucose . Glucokinase differs from hexokinase in : a- Requiring a much higher glucose concentration for half saturation . Thus , glucokinase functions only when the intracellular concentration of glucose * the hepatocyte is elevated , such as during the brief period following consumption of a carbohydraterich meal , when high level of glucose are delivered to the liver via the portal vein ( blood glucose equilibrates rapidly across the membrane of the hepatocyte ) . b- Glucokinase has high ** allowing the liver to effectively remove this flood of glucose from the portal blood . This prevents large amounts of glucose from entering the systemic circulation following a carbohydrate rich meal , and thus minimizes hyperglycemia during the absorptive period . c- Glucokinase levels are increased by carbohydrate-rich diets and bu insulin . d- Glucokinase is not inhibited by glucose 6-phosphate . 6 Glucokinase 1- Only in liver and pancreatic islets . 2- Acts on glucose . 3- Low affinity to glucose ( Km ↑ ) and high V max . 4- Not inhibited by by G-6-p . 5- Level depends on glucose conc. Hexokinase 1- In all tissues except liver and pancreatic islets . 2- Glucose , fructose and galactose. 3- High affinity to glucose ( Km ↓ ) and low V max . 4- Inhibited by G-6-p . 5- No change on fasting or high CHO diet . 6- No change in diabetes . 7- Not induced by insulin . 6- Level decreased in diabetes . 7- Synthesis induced by insulin . B. Isomerization of glucose 6-phosphate : It is catalyzed by phosphoglucose isomerase . The reaction in readily reversible and is not a rate-limiting or regulate step . C- Phosphorylation of fructose 6-phosphate : This irreversible phosphorylation reaction catalyzed by phosphofructokinase I ( PEK –1 ) . PEK-1 reaction is the rate – limiting step in glycolysis . 1- PEK-1 is inhibited allosterically by elevated levels of ATP , which act as as “energy rich “ signal indicating an abundance of highenergy compounds . 2- Elevated levels of citrate ( starting of citric acid cycle ) also inhibit PEK-I . 3- Conversely , PEK-1 is activated allosterically by high concentration of AMP , which signal that the cell’s energy stores are depleted . 4- Fructose 2 , 6 –bis-phosphate is the most potent activator of PEK the reciprocal actions of fructose 2,6-bisphosphate on glycolysis and gluconeogenesis ensure that both pathways are not fully active at the same time . Stage II A- Cleqavage of fructose 1 , 6-biphosphate : Aldolase A cleaves fructose 1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate . The reaction is reversible and is not regulated . B- Isomerization dihydroxyacetone phosphate Triose phosphate isomerase interconverts dihydroxyacetone phosphate and glyceraldehyde 3-phosphate . Dihydroxyacetone phosphate must be isomerized to glyceraldehyde 3-phosphate for further metabolism in the glycolic sequence . This isomerization results in the production of two 7 molecules of glyceraldehyde 3-phosphate from the cleavage products of fructose 1,6-bisphosphate . Stage III [A] Oxidation of glyceraldehyde 3-phosphate The conversion of two moles of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate by glyceraldehyde 3-phosphate dehydrogenase is the first oxidation – reduction reaction of glycolysis . [ Note : Because there is only a limited amount of NAD+ in the cell , the NADH formed must be reoxidized to NAD+ for glycolysis to continue ] . Two major mechanisms for oxidizing NADH : [1] The NAD-linked conversion of pyruvate to lactate ( anaerobic ) . [2] Oxidation via the respiratory chain ( generating 2 x 3 ATP ) . The oxidation of the aldehyde group of glyceraldehyde 3-phosphate to a carboxyl group is coupled to the attachment of pi to the carboxyl group . The high-energy phosphate group at carbon 1 of 1,3 bisphosphoglycerate conserves much of the free energy produced by the oxidation of glyceraldehyde 3-phosphate . [B] i- Substrate-level phosphorylation : The high-energy phosphate group in 1,3-bisphosphoglycerate ( two moles ) is used to synthesis 2 ATP from 2 ADP in a reaction catalyzed by phosphoglycerate kinase . This reaction is reversible ( unlike other reactions of kinase ) . This is an example of substrate –level phosphorylation in which the production of a high energy phosphate is coupled directly to the oxidation of a substrate , instead of resulting from oxidative phosphorylation via the electron transport chain . ii- 1,3-Bisphosphoglycerate is converted to 2,3-bisphosphoglycerate ( 2,3-BpG ) by the action of bisphosphoglycerate mutase . 2,3-BPG , which is found in only trace amounts in most ceolls , is present at high concentration in red blood cells . 2,3-BPG is hydrolyzed by a phosphate to 3-phpsphoglycerate , which is also an intermediate in glycolysis . In the red blood cell , glycolysis is modified of these “shunt” reactions . [C] I- Shift of the phosphate group from carbon 3 to carbon 2 : This reaction is catalysed by phosphglycerate mutasde which is freely reversible . ii- Dehydration of 2-phosphoglycerate : The dehydration of 2-phosphoglycerate by enolase redistributes the energy within the 2-phosphoglycerate molecules , resulting in the formation of phosphoenolpyruvate ( PEP ) , which contains a high energy enol phosphate , The reaction is reversible despite the high energy nature of the product . 8 iii- Formation of pyruvate ( substrate-level phosphorylation ) : The conversion of PEP to pyruvate is catalyzed by pyruvate kinase , the third irriversible reaction of glycolysis . The equilibrium of the pyruvate kinase reaction favors the formation of ATP ( 2 ATP from 2 mols ) . [ This is a second example of substrate-level phosphorylation ] . In liver, Pyruvate kinase is activated by fructose 1,6-bisphosphate the product of the phosphofructokinase reaction . This feed-forward ( instead of the more usual feed back ) regulation has the effect of linking the two kinase activities : increased phosphofructokinase activity , resulting in elevated levels of fructose 1,6-bisphosphate activities pyruvate kinase . Stage IV Reduction of pyruvate to lactate ( anaerobic ) : Lactate , formed by the action of lactate dehydrogenase ( LHD ) is the final product of anaerobic glycolysis in eukaryotic cells . The formation of lactate is the major fate for pyruvate in red blood cells , lens and cornea of the eye , Kidney medulla , testes , and leukocytes . The significance of pyruvate to lactate transfer under an aerobic conditions : Under aerobic condition NADHH produced from the reaction of glyceraldehyde 3-p dehydrogenase ( stage III ) is back transferred to NAD+ by losing its 2 H to the mitochondria respiratory chain ( forming 3 ATP ) . However under anaerobic conditions NADHH , instead , loses its 2H to pyruvate via LDH transferring it to lactate , thus oxidized NAD+ is regenerated . This permits glycolysis to proceed even under anaerobic conditions . 1. Lactate formation in muscle : In exercising skeletal muscle , NADH production ( by glyceraldehyde 3-phosphate dehydrogenase and by the three NAD+ -linked dehydrogenase of the citric acid cycle ) exceeds the oxidative capacity of the respiratory chain . This results in an elevated NADH/NAD+ ratio favoring reduction of pyruvate to lactate . Therefore, during intense exercise , lactate accumulates in muscle , causing a drop in the intracellular PH, potentially resulting in cramps . Much of this lactate eventually diffuses into the blood stream . 2. Lactate consumption : In liver and heart , the ratio of NADH/NAD+ is lower than in exercising muscle . These tissues oxidize lactate ( obtained from the blood ) to pyruvate . In the liver , pyruvate is either converted to glucose . by gluconeogensis or oxidized in the TCA cycle . Heart muscle exclusively oxidize lactate to CO2 and H2o via the citric acid cycle being highly supplied with oxygen . 9 3. In RBCs : Even under aerobic conditions , glycolysis in RBCs usually terminates with lactate , since RBCs has no mitochondria that contains the enzymes responsuble of aerobic oxidation of pyruvate ( citric acid cycle enzymes ) . The next figure ( page 31 ) shows the collective reactions of glycolysis : ALTERNATE FATES OF PYRUVATE A. Oxidative decarboxylation of pyruvate Pyruvate resulted from aerobic glycolysis in cytosol enters the mitochondria where it is oxidatively decarboxylation by pyruvate dehydrogenase complex . This is an important path way in tissue with high oxidative capacity , such as cardiac muscle , pyruvate dehydrogenase irreversibly converts pyruvate , the end product of glycolysis , into acetyl COA , a major fuel for the citric acid cycle . B- Carboxylation of pyruvate to oxaloacetate Carboxylation of pyruvate to oxaloacetate (OAA) by pyruvate carboxylase is a biotin-dependent reaction . This reaction is important because it replenishes the citric acid cycle intermediates and provides substrate for gluconeogenesis ( see p. 99 ) ENERGY YIELD OF GLYCOLYSIS Reaction ATP (*p) formed per mole glucose 1- Hexokinase ( Glucokinase ) -1 ATP 2- PFK-1 -1ATP 3- Glyceraldehyde 3p dehydrogenase ( aerobic ) +2 x 3 ATP 4- Phosphoglycerate kinase ( substrate level ) +2 x 1 ATP 5- Pyruvate kinase ( substrate level ) +2 x 1 ATP TOTAL Aerobic = 8 ATP Anaerobic = 2ATP REGULATION OF GLYCOLYSIS ENZYME Activators 1- Hexokinase Insulin ( inducer ) 2- PEK-1 AMP , F2,6bisphosphate 3- Pyruvate kinase F1,6-bisphosphate Inhibitors G 6-P High ATP , Citrate ATP ATP HORMONAL REGULATION OF GLYCOLYSIS Glycolytic enzymes - Consumption of a meal rich in carbohydrate or administration of insuliun initiates an increase in the amount of glucokinase . - These changes reflect an increase in gene transcription , resulting in increased enzyme synthesis . 10 - High activity of these three enzymes favors the conversion of glucose to pyruvate , a characteristic of the well-fed state . - Conversely , gene transcription and synthesis of glucokinase , phosphofructokinase , and pyruvate kinase are decreased when plasma glucagon is high and insulin is low , for example , as seen in starvation or diabetes . CITRIC ACID CYCLE KREBS CYCLE TRICARBOYLIC ACID CYCLE ( TCA ) The citric acid cycle plays several roles in metabolism : *Its central function is the oxidation of acetyl COA to CO2 and H2o . Acetyl CoA is derived from the metabolism of fuel molecules such as amino acids , fatty acids , and crbohydrates . This oxidation accounts for about two thirds of the total oxygen consumption and ATP production in most animals , including humans . * The citric acid cycle also participate in a number of important synthetic reactions . For example , the cycle functions in the formation of glucose from the carbon skeleton of amino acids and provides building blocks for heme synthesis . * The cycle occurs totally in the mitochondrial matrix and is therefore in close proximity to reactions of oxidative phosphorylation . REACTIONS OF THE CITRIC ACID CYCLE In THE CITRIC ACID CYCLE ** is first condensed with acetone (acetyl COA) , then regenerated as the cycle is completed , these reactions should not be viewed only as a closed circle , but rather like a traffic circle with compounds entering and leaving as required . 1] Oxidative decarboxylation of pyruvate pyruvate dehydrogenase complex ( PDH ) is a multienzyme complex located in the mitochondrial matrix . It converts pyruvate , the end product of aerobic glycolysis into acetyl COA , a major fuel for the citric acid cycle . the irreversibility of the reaction precludes the formation of the pyruvate from acetyl COA and explains why glucose cannot be formed from acetyl COA ( derived from fatty acids oxidation ) in gluconeogenesis . A- Component enzymes : The pyruvate dehydrogenase complex is a multimolecular aggregate of three enzymes : pyruvate Decarboxylase , dihydrolipoyl trans acetylase , and dihydrolipoyl dehydrogenase . Each catalyzes a part of the overall reaction . their 11 physical association links the reaction in proper sequences without the release of intermediates . B- Coenzymes : The PDH complex contains five coenzymes : thiamine pyrophosphate ( TTP ) , lipoic acid , COASH , FAD and NAD . They act as a carriers or oxidants for the intermediates of the reaction : C- Regulation : PDH is inhibited by product ( acetyl CoA, NADHH and ATP ) . it is activated by insulin ( in adipose tissue ) . 2- Synthesis of citrate from acetyl CoA and oxaloacetate The condensation of acetyl CoA and oxaloacetate is catalyzed by citrate synthase . this indol condensation has an equilibrium far in direction of citrate synthase ( irreversible ) . Citrate synthase is inhibited by ATP , NADH , succinyl CoA , and acetyl CoA derrivatives of fatty acids . 3- Isomerization of citrate Citrate is isomerized to isocitrate by aconitase . This conversion takes place in two steps : dehydration to cis-aconitate , and rehydration to isocitrate This reaction is inhibited by fluoroacetate . 4- Oxidation and decarboxylation of isocitrate Isocitrate dehydrogenase catalyses the oxidative decarboxylation of isocitrate to α-ketoglutarate ( oxalosuccinate is formed as intermediate ) , yielding the first of three NADH molecules produced by the cycle , and the first release of CO2 . This is one of the rate-limiting steps of the citric acid cycle . The enzyme is activated by ADP . Elevated levels of mitochondrial ADP signal a need for generation of more high-energy phosphate ( ATP ) . The enzyme is inhibited by ATP and NADH , whose levels are elevated when the cell has abundant energy stores . Another form of isocitrate dehydrogenase is also present in cytosol and is NADP –linked enzyme instead of NAD . 5- Oxidative decarboxylation of α-ketoglutarate Conversion of α-ketoglutarate to succinyl CoA is catalysed by the α-ketoglutarate dehydrogenase complex . The mechanism of the oxidation decarboxylation is similar to that used for the conversion of pyruvate to acetyl CoA . The reaction release the second Co2 and produces the second NADH of the cycle . This reaction is irreversible . Coenzyme : The coenzymes required are thiamine pyrophosphate , lipoic acid , FAD , NAD+ , and CoA . Each functions in the cataalytic mechanism in a way analogous to that described for pyruvate dehydrogenase complex . 12 6- Cleavage of succinyl CoA Succinate thiokinase cleaves the high-energy thioester bond of succinyl CoA . This reaction is coupled to phosphorylation of GDP to GTP . The energy content of GTP is the same as that of ATP , and the two nucleotides are interconvertible by the nucleoside diphosphate kinase reaction : Thus ATO formed from GTP in this reaction is an example of substrate level phosphorylation . N.B. succinyl CoA also formed from fatty acids with an old number of carbon atoms and from propionyl CoA derived from the mechanism of branched-chain amino acids . Succinyl CoA is used in the biosynthesis of heme . 7- Oxidation of succinate Succinate is oxidized to fumarate by succinate dehydrogenase , producing the reduced coenzyme FADH2 . FAD , rather than NAD+ , is the electron acceptor because the reducing power of succinate is not sufficient to reduce NAD+ . Malonate , a dicarboxylic acid that is a structural analog of succinate , competitively inhibits succinate dehydrogenase . 8- Hydration of fumarate Fumarate is hydrated to malate in a freely reversible reaction catalysed by fumarase . 9- Oxidation of malate Malate is oxidized to oxaloacetate by malate dehydrogenase . This reaction produces the third and final NADH of the cycle . Summary of reactions 1. Two carbon atoms enter the cycle as acetyl CoA and leave as Co2 . 2. The cycle does not involve net consumption or production of oxaloacetate or of any other intermediate . 3. Four pairs of electrons are transferred during one turn of the cycle : three pairs of electrons reducing NAD+ to NADH and one pair reducing FAD to FADH2 . Yield of ATP Oxidation of one NADH by the electron transport chain leads to formation of three ATP , whereas oxidation of FADH2 yields two ATP . 13 Energy yield from oxidation of one mole of acetyl CoA via TCA cycle : Enzyme No of ATP produced Isocitrate dehydrogenase ( NADH ) 3 ATP 3 ATP a-ketoglutarate dehydrogenase (NADH) Succinate thiokinase (substrate 1 ATP level ) Succinate dehydrogenase (FADH2) 2 ATP Malate dehydrogenase (NADH) 3 ATP 12 ATP TOTAL Oxidation of one mole of glucose under aerobic condition ***** Thus one mole of glucose oxidized completely under aerobic condition gives 38 ATP while under anaerobic it is converted to lactate yielding 2 ATP . Pasteur Effect : Pasteur observed that Yeast cells consumed glucose much more slowly under aerobic than under anaerobic conditions . This is explained by that , under anaerobic condition . The large production of ATP ( via citric acid cycle ) inhibits glycolysis by inhibiting PEK-1 pyruvate kinase , this leads to accumulation of G6 –p which in turn , inhibits further uptake of glucose by cells due to allosteric inhibition of hexokinase .” VITAMINS PLAY KEY ROLES IN THE CITRIC ACID CYCLE *********** functioning of the citric acid cycle . They are : [1] Riboflavin , in the form of flavin adenine dinucleotide ( FAD ) , a cofactor in the α-ketoglutarate dehydrogenase complex and in succinate dehydrogenase . [2] Niacin , in the form of nicotinamide adenine dinucleotide ( NAD ) , the coenzyme for three dehydrogenases in the cycle , isocitrate dehydrogenase , α-ketoglutarate dehydrogenase , and malate dehydrogenase . [3] Thiamin , ( vitamin B1 ) , as thiamin diphosphate , the coenzyme for decarboxylation in the α-ketoglutarate dehydrogenase reaction . 14 [4] Pantothenic acid , as part of coenzyme A, the cofactor attached to “active” carboxylic acid residues such as acetyl-CoA and succinyl-CoA . THE CITRIC ACID CYCLE PLAYS A PIVOTAL ROLE IN METABOLISM The amphibolic Role Of Citric Acid Cycle Some metabolic pathways end in a constituent of the citric acid cycle while other pathways originate from the cycle . These pathways concern the processes of gluconeogenesis , transmination , deamination , and fatty acid synthesis . Therefore , the citric acid cycle plays roles in both oxidative synthetic processes ; i.e, it is amphibolic . These roles are summarized [1] Role of the Cyclic in Gluconeogenesis : All major members of the cycle , from citrate to oxaloacetate , are potentially glucongenic , since they can give rise to anet production of glucose in the liver or kidney , the organs that contain a complete set of enzymes necessary for gluconeogenesis . The key enzyme that facilitates the net transfer out of the cycle into the main pathway of gluconeogenesis is phosphoenolpyruvate carboxykinase ( PEP carboxykinase ) , which catalyzes the decarboxylation of oxaloacetate to phosphoenolpyruvate , GTP acting as the source of high-energy phosphate ( will be described in gluconeogenesis ) . [2] Role in Transamination : i- Pyruvate Alanine ii- Oxaloacetate Aspartate ii- α-ketoglutarate Glutamate [3] Final pathway for the oxidation acids e.g. i- Glucose pyruvate ii- Fatty acids β-oxidation iii- Serine , Cystine , Glycine iv- Tryosine , Phenylalanine v- Methionine , valine [4] Synthetic pathways e.g. i- Succinyl CoA + Glycine ii- Citrate Acetyl CoA of Glucose , Fatty acids & Amino Acetyl CoA . Acetyl CoA . Acetyl CoA . pyruvate Fumarate . Succinyl CoA . Heme . Fatty acids . Hexose Monophosphate Shunt ( HMP ) “ Pentose Phosphate Pathway ( PPP ) “ ***** Phosphate pathway or phosphogpuconate pathway ) : 15 It contains of two irreversible oxidative reactions , followed by a series of reversible sugar-phosphate interconversion . No ATP is directly consumed or produced in the cycle . Carbon 1 of glucose 6-phosphate is released as Co2 , and two NADH are produced for each glucose 6-phosphate entering the oxidative part of the pathway . Unlike glycolysis or the citric acid cycle in which the direction of the reaction is well defined , the interconversion reactions of the HMP can function in several different directions . The rate and direction of the reactions at any given time are determined by the supply of and demand far intermediates in the cycle . The HMP occurs in the cytosol of the cell . Metabolic significance of HMP shunt ( functions ) : The pathway provides a major portion of the cell’s NADPH , which functions as a biochemical reactant . It is particularly important in liver and mammary glands , which are active in the biosynth esis of fatty acids , and in the adrenal cortex which is active in the NADPH-dependent synthesis of steroids . The HMP also produces ribose-phosphate , required for biosythesis of nucleotides and provides a mechanism for the metabolic utilization of five-carbon sugars ingested as food . Reactions of HMP A] OXIDATIVE REACTIONS The oxidative portion of the HMP consists of three reactions that lead to the formation of ribulose 50phosphate , Co2 , and two molecules of NADH for each molecule of glucose 6-phosphate oxidized : 1- Dehydrogenation of glucose 6-phosphate : Glucose 6-phosphate dehydrogenase ( G6PD ) catalyzes an irreversible oxidation of glucose 6-phosphate tp 6phosphogluconolactone in a reaction that is specific for NADP as coenzyme . 2- Hydrolysis of 6-phosphogluconolactone and formation of ribulose 5-phosphate 6-Phosphogluconolactone is hydrolyzed by Phosphogluconolactone hydrolase to 6-phosphogluconate . The reaction is irreversible and is not rate-limiting . 16 The subsequent oxidative decarboxylation of 6-phosphogluconate is catalyzed 6-phosphogluconate dehydrogenase . This irreversible reaction produces a pentose sugar phosphate ( ribulose 5-phosphate ) , Co2 ( from carbon 1 of glucose ) , and a second molecule of NADPH . B] NONOXIDATIVE REACTIONS `The nonoxidative reactions of the pentose phosphate pathway catalze the interconversion of three- , four- , five- and seven-carbon sugars . These reactions permit ribulose 5-phosphate ( produced by the oxidative portion of the pathway ) to be converted either to ribose 5phosphate ( needed for nucleotide synthesis ) or to intermediates of glycolysis , such as fructose 6-phosphate and glyceraldehyde 3-phosphate . Thus the HMP is not an isolated repetive cycle , but is integrated with glycolysis . The only coenzyme required in the nonoxidative pathway is thiamine pyrophosphate ( TPP ) in the transketolase reaction . Interconversion between HMP and Glycolysis 1- Conversion of pentose phosphate to intermediates of glycolysis Many cells that carry out reductive biosynthetic reactions have a greate need for NADPH than ribose 5-phosphate . In this case , transketolase and transaldolase convert the ribose 5-phosphate produced as an end product of the oxidative reaction to glyceraldehyde 3-phosphate and fructose 6-phosphate , which are intermediates of glycolysis . 2- Formation of ribose 5-phosphate from intermediates of glycolysis Under conditions where the demand for pentoses for incorporation into nucleotides and nucleic acids is greater than the need for NADPH, the nonoxidative reactions can provide the biosynthesis of ribose 5-phosphate from fructose 6-phosphate in the absence of the oxidative steps . Metabolic roles of reduced NADP ( NADPH ) produced from HMP A. Reductive biosynthesis NADPH can be thought of as a high-energy molecule , much in the same way as NADH . However , the electron of NADPH are designed for use in reductive biosynthesis , rather than for transfer to oxygen as in the case wit NADH . Thus in the metabolic transformation of HMP , part of the energy of glucose 6-phosphate is conserved in NADPH , a molecule that can be used in reactions requiring a high electron-potential electron donor For example , NADPH is used as a source of electrons for the biosynthesis of fatty acids and steroids as cholestrol and steroid hormones. *** Reduction of hydrogen peroxide 17 Hydrogen peroxide is one of a family of reactive oxygen intermediates that are formed from the partial reduction of molecular oxygen . These compounds are formed continuously as by-products of aerobic metabolism and through reactions with drugs and environmental toxins . They are highly reactive and can cause serious chemical damage to DNA , proteins , and unsaturated lipids . Reactive oxygen intermediates have been implicated in a number of pathogenic processes , including reperfusion injury , cancer , inflammatory disease , and aging . The cell has several protective mechanisms that serve to minimize the toxic potential of these compounds . Enzymes that catalyze antioxidant reactions : Reduced glutathione , a tripeptide-thiol ( **glutamylcysteinylglycine ) present in most cells , can chemically detoxify hydrogen peroxide . This reaction , catalyzed by glutathione peroxidase , forms oxidized glutathione , which no longer has protective properties . The cell regenerates reduced glutathione in a reaction catalyzed by glutathione . Reductase using NADPH as a source of reducing electrons . Thus NADPH indirectly provides electrons for the reduction of hydrogen peroxide . GLUCOSE 6-P DEHYDROGENASE DEFICIENCY G6PD deficiency is an inhereted disease characterized by hemolytic anemia caused by the inability to detoxify oxidizing agents , G6PD . Deficiency is the most common disease – producing enzyme abnormality in humans , affecting more than 200 milion individuals world wide . This X-linked enzyme deficiency is , in fact , a family of deficiencies caused by over 400 different mutations in the gene coding for G6PD . only some of these mutations cause clinical symptoms . The life span of many individuals with G6PD deficiency is some what shortened as a result of complications arising from chronic hemolysis . Role of G6Pd in red blood cells Diminished G6PD activity impairs the ability to form NADPH that is essential in the detoxification of free radicals and peroxides formed within the cell ( figure 10.9 ) . Although the deficiency occurs in all cells of the affected individual , it is most severe in erythrocytes where the HMP provides the only means of generating NADPH . other tissues have alternative sources for NADPH production . 18 The erythrocyte has no nucleus or ribosomes and cannot renew its supply of the enzyme . Thus , red blood cells are particularly vulnerable to enzyme variants with diminished stability . Precipitating factors in G6PD deficiency Most individuals who have inherited one of the many G6PD mutations do not show clinical manifestations . However , some patients with G6PD deficiency develop hemolytic anemia if they are treated with n oxidant drug , ingest fava beans , or contact a severe infection . 1. Oxidant drugs : Commonly used drugs that produce hemolytic anemia in patients with G6PD deficiency are best remembered from the mnemonic AAA -= Antibiotics ( for example suifamethoxazole ) , Antimalarials ( for example , primaquine ) and Antipyretics ( for example , acetanilid , but not aspirin or acetaminophen ) . 2. Flavin : Some forms of G6PD deficiency , for example the Mediterranean variant , are particularly susceptible to the hemolytic effect of the fava bean , a dietary staple in the Mediterranean region . 3. Infection : Infection is the most common precipetating factor of hemolysis in G6PD deficiency . The inflammatory response to infection results in the generation of free radicals in macrophages , which can diffuse into the red blood cells and cause oxidative damage. 4. Neonatal jaundice : Individuals with G6PD deficiency may experience neinatal jaundice , which may result from impaired hepatic catabolism or increased production of bilirubin . GLYCOGEN METABOLISM Glycogenesis & Glycogenolysis A constant source of blood glucose is an absolute requirement for human life . Glucose is the preferred energy source of the brain and the required energy source for cells with few or no mitochondria , such as mature erythrocytes . Glucose is also essential as an energy source in exercising muscle , where it is the substrate for anaerobic glycolysis . Blood glucose can be obtained from three primary sources : diet , degradation of glycogen , and gluconeogenesis . The body has developed mechanisms for storing a supply of glucose in a rapidly mobilizable form – glycogen . In the absence of a dietary source of glucose , glucose is rapidly released from liver glycogen . 19 Similarly , muscle glycogen is extensively degraded in working muscle . when glycogen stores are depleted , specify tissues synthesize glucose do novo , using amino acids from the body’s proteins as the primary source of carbons for the gluconeogenic pathway . Functions of Glycogen : The main stores of glycogen in the body are found in skeletal muscle and liver , although most other cells may store minute amounts . The function of muscle glycogen is to serve as a fuel reserve for the synthesis of ATP during muscle contraction . That of liver glycogen is to maintain the blood glucose concentration , particularly during the early stages of a fast . Amounts of liver and muscle glycogen Approximately 400 g of glycogen makes up 1% to 2% of the fresh weight of resting muscle , and approximately 100 g of glycogen makes up 6% to 8% of the fresh weight of well-fed adult liver . Thus glycogen conce3ntration in liver is greater than muscle . However , the total amount of glycogen in muscle is greater than liver ( since the total muscle mass is much greater than liver ) Structure of glycogen Glycogen exists in discrete cytoplasmic granules that contain most of the enzymes necessary for glycogen synthesis and degradation . Glycogen is a branched-chain homopolysaccharides made exclusively from α-D-glucose . The primary glycosidic bond in an **-1,4 linkage . After every eight to ten glycosyl residues , there is a branch containing an **-1,6 linkage . GLYCOGENESIS ( Synthesis of Glycogen ) - Glycogen is synthesized from molecules of α -D-glucose . - The process occurs in the cytosol , and requires energy supplied by ATP ( for the phosphorylation of glucose ) and uridine triphosphate (UTP) . A. Synthesis of UDP –glucose α-D-Glucose attached to uridine diphosphate ( UDP ) is the source of all the glucosyl residues that are added to the growing glycogen molecule UDP –glucose is synthesized from glucose 1-phosphate and UTP by UDP-glucose pyrophosphorylase . Note : Glucose 6-phosphate is converted to glucose 1-phosphate by phosphoglucomutase . 20 B. Synthesis of a primer to initiate glycogen synthesis Glycogen synthase is responsible for making the α-1,4 linkage in glycogen . This enzyme cannot initiate chain synthesis using free glucose as an acceptor of a molecule of glucose from UDP –glucose . Instead , it can only elongate already existing chains of glucose . Therefore , a fragment of glycogen can serve as a primer in cells whose glycogen stores are not totally depleted . In the absence of a glycogen fragment , a specific protein , called glycogenin , can serve as an acceptor of glucose residues . The hydroxyl group of a specific tyrosine side chain serves as the site at which the initial glucosyl unit is attached . Transfer of the first molecule of glucose from UDP –glucose to glycogennin in catalyzed by glycogen initiator synthase . C. Elongation of glycogen chains by glycogen synthase Elongation of a glycogen chain involves the transfer of glucose from UDP-glucose to the non reducing end of the growing chain , forming a new glycosidic bond between the anomeric hydroxyl of carbon 1 of the activated glucose and carbon 4 of the accepting glucosyl residue . The enzyme responsible for making the **-1,4 linkage in glycogen is glycogen synthase . D. Formation of branches in glycogen Glycogen is a highly branched , tree like structure , that i8s far more soluble than its unbranched amylose cousin . Branching also increases the number of non reducing ends to which new glucosyl residues can be added , thereby greatly accelerating the rate at which glycogen synthesis and degradation can occur , and dramatically increasing the size of the molecule . 1. Amylo –( 1,4- 1,6 ) –transglycosylase ( branching enzyme ) transfers a chain of five to eight glucosyl residues from the non reducing end of the glycogen chain to another residue on the chain and attaches it by an α -1,6 linkage . The resulting new , non reducing end can now be further elongated by glycogen synthase . 2. Synthesis of additional branches : After elongation of these two ends has been accomplished by glycogen synthase , their terminal five to eight glucosyl residues can be removed and utilized to make further branches . GLYCOGENOLYSIS ( Degradation of Glycogen ) The degradation pathway that mobilizes stored glycogen in liver and skeletal muscle is not a reversed of the synthetic reactions . Instead an independent set of enzymes is required . When glycogen is degraded , the primary product is glucose 1-phosphate , obtained by breaking α -1,4 21 glycosidic bonds . In addition , free glucose is released from each α - 1,6linked glucosyl residue . Shortening of chains Glycogen phosphorylase cleaves the *-1,4 glycosidic bonds between the glucosyl residues at the non-reducing ends of the glycogen chains by simple phosphorolysis . Glycogen phosphorylase is a phosphotransferase that sequentially degrades the glycogen chains at their non-reducing ends until four glucosyl units remain on each chain before a branch point . The resulting structure is called a limit dextrin , and phosphorylase cannot degrade it any further ( Figure 13.7 ) . B. Removal of branches Branches are removed debranching enzyme ( amylo-**- ( 1,6 ) – glucosidase Conversion of glucose 1-phosphate to glucose 6-phosphate Glucose 1-phosphate , produced by glycogen phosphorylase , is converted to glucose 6-phosphate by phosphoglucomutase . In liver and kidney : The enzyme glucose 6-phosphate is oresent , which trasfers glucose 6-p tp glucose that can diffuse from the cell to the blood to maintain blood glucose level . Thus the end product of glycogenolysis in liver is glucose . In Muscle : glucose 6-phosphatase is absent , thus G6P resulted from glycogenolysis in mu8scle is oxidized to lactate via glycolysis . REGULATION OF GLUCOGENSIS AND GLYCOGENOLYSIS Because of the important of maintaining blood glucose levels , the synthesis and degradation og its storage form , glycogen , are tightly regulated . In the liver , glycogen synthesis accelerates during periods when the body has been well fed , whereas glycogen degradation accelerates during periods of fasting . In skeletal muscle , glycogen degradation occurs during active exercise , and accumulation begins as soon as muscle is again at rest . Both synthesis and degradation of glycogen are controlled through a complex mechanism involving insulin , glucagon and epinephrine . These hormones initiate processes that result in the control; of several sets of enzymes . 1. The binding of glucagon to liver cells stimulates glycogenolysis and inhibits glycogenesis , As blood glucose levels drop in the hours after a meal , glucagon ensures that glucose will be released intl blood stream . 22 2. As a result of glucagon’s binding to its receptor , adenylate cyclase ( a cell membrane enzyme ) is stimulated to convert ATP to the second messenger cAMP . cAMP then initiates a reaction cascade that amplifies the original signal . 3. Within seconds a few glucagon molecules cause the release of thousands of glucose molecules . 4. The binding of insulin to receptors on the surface of several cell types stimulate glycogenesis and inhibits glycogenolysis . 5. The mechanism of insulin’s action is poorly understood . In addition to increasing the rate of glucose uptake into several types of cell ( but not liver or brain cells ) , insulin inhibits several enzymes that are activated by the cAMP reaction cascade . 6. Emotional or physical stress results in the release of epinephrine from the adrenal medulla . 7. Epinephrine affects glycagon metabolism in liver and muscle , while only glucagon effect is on liver . This is because of the presence of epinephrine receptors in both liver and muscle while glucagon receptors are present only in liver ceels . 8. Epinephrine promotes glycogenolysis and inhibits glycogenesis . In emergency situations when epinephrine is released in relatively large quantities , the resulting massive production of glucose provides the energy required to manage the situation . 9. This phenomena is referred to as the “ flight – or – fight “ response . 10.Epinephrine initiates the process by activating adenylate cyclase in liver and muscle cells . 11.Two other second messengers , calcium ions and inositol triphosphate are also belived to be involved in epinephrine’s action . Glycogen synthase and glycogen phosphorylase exist in Active and inactive conformations . The interconversion between these forms is effected by covalent modification . 1. The active form of glycogen synthase , known as the ( independent ) 1 form is converted to the inactive or D ( dependent ) form by phosphorylation . 2. In contrast , the inactive form of glycogen phosphorylase (phosphorylase b) is converted to the active form (phosphorylase a) by the phosphorylation of a specific serine residue . The phosphorylating enzyme is called phosphorylase kinase . 3. Phosphorylation of both glycogen synthase and phosphorylase kinase is catalyzed by a protein kinase , which in turn is activated by cAMP . 23 4. Glycogen synthesis occurs when glycogen synthase and glycogen phosphorylase have been dephosphorylated . This conversion is catalyzed by phosphoprotein phosphatase . 5. Phosphoprotein phosphatase also inactivates phosphorylase kinase . GLUCONEOGENESIS It is the pathway responsible for the conversion of non carbohydrates to glucose or glucogen . The major substrates for gluconeogenesis are : 1- pyruvate & lactate ( the product of anaerobic glycolysis in muscles andRBCs ) . 2- Gluconeogenic amino acids . 3- Glycerol resulted from lipolysis of triacetyl glycerols ( fat ) in adipose tissue . 4- Propionate ( in ruminants only ) . Gluconeogenesis occurs mainly in liver and kidney . Some tissues , such as the brain , red blood cells , kidney medulla , lens and cornea of the eye , testes , and exercising muscle , require a continous supply of glucose as a metabolic fuel . Liver glycogen can meet these needs for only 10 to 18 hours in the absence of dietary intake of carbohydrate . During a prolonged fast , hepatic glycogen stores are depleted and glucose is formed from precursors such as lactate , pyruvate , glycerol ( derived from the backbone of triacylglycerols ) and *** ) derived from amino acid catabolism ) . The formation of glucose does not occur by a simple reversal of glycolysis , because the overall equilibrium of glycolysis strongly favors pyruvate formation . Instead , glucose is synthesized by a special pathway , gluconeogenesis . Approximately 90% of gluconeogenesis occurs in ths liver . whereas kidneys provide 10% of newly synthesized glucose molecules . The Kidneys thus play a minor role except during prolonged starvation , when they become major glucose producing organs . SUBSTRATES FOR GLUCONEOGENESIS Gluconeogenesis precursors are molecules that can give rise to a net synthesis of glucose . They include all the intermediates of glycolysis and the citric acid cycle . Glycerol , lactate , and the ***keto acid obtained from deamination of glucogenic amino acids are the most important gluconeogenic precursors . REACTIONS UNIQUE TO GLUCONEOGENESIS 24 Seven of the reactions of glycolysis are reversible and are used in the synthesis of glucose from lactate or pyruvate . However , three of the reactions are irreversible and must be circumstances and must be circumvented by four alternate reactions that energetically favor the synthesis of glucose . These irreversible reactions ( energy barriers or exergonic reactions ) that obstruct a simple reversal of glycolysis are : 1- From PEP to pyruvate ( pyruvate kinase ) . 2- From fructose 6-phosphate to fructose 1,6-bisphosphate ( PEK-1 ) . 3- From glucose 6-phosphate to glucose ( Hexokinase or Glucokinase ). 4- From glucose 1-phosphate to glycogen . I- Gluconeogenesis from Lactate and Pyruvate : 1- Conversion of Pyruvate to phosphoenol pyruvate ( PEP ) : A. Carboxylation of pyruvate The first “roadblock” to overcome in the synthesis of glucose from pyruvate is the irreversible conversion of pyruvate to PEP by pyruvate kinase . In gluconeogenesis , pyruvate is first carboxylated by pyruvate carboxylase to oxaloacetate ( OAA ) , which is then converted to PEP by the action of PEP-carboxykinase . Note : pyruvate carboxylase is found in the mitochondria of liver and kidney cells , but not of muscle . ] pyruvate carboxylase contains biotin as co-carboxylase . pyruvate carboxylase is allosterically activated by acetyl CoA . Elevated levels of acetyl CoA may signal one of several metabolic states in which the increased synthesis of oxaloacetate is required . For example , this may occur during starvation where OAA is used for the synthesis of glucose by gluconeogenesis . B. Transport of oxaloacetate to the cytosol Oxaloacetate , formed in mitochondria , must enter the cytosol where the other enzymes of gluconeogenesis are located . However , oxaloacetate is unable to cross the inner mitochondrial membrane directly ; but it can be transported by either : 1- Reduction to malate ( by malate deyhydrogenase ) , which can be transported to oxaloacetate . 2- Trasferred to citrate ( by citrate synthase ) , then citrate transported to cytoplasm and again transferred to oxaloacetate by enzyme ATP –citrate lyase . 3- Transamination to aspartate . C. Decarboxylation of cytosolic oxaloacetate 25 Oxaloacetate , in cytosol , is decarboxylated and phosphorylated to PEP by PEP-carboxykinase . High energy phosphate in the form of GTP or ITP is required in the reaction , and Co2 is liberated . PEP can be transferred easily to fructose 1,6-bisphosphate by the reversal of glycolic reactions . 2- Dephosphorylation of fructose 1.6-bisphosphate : Hydrolysis of fructose 1.6-bisphosphate by fructose 1.6bisphosphate by passes the irreversible phosphofructokinase-1 reaction and provides an energetically favorable pathway for the formation of fructose -6phosphate This reaction is an important regulatory site of gluconeogenesis . fructose 1.6bisphosphate occurs in liver and kidney . 3- Dephosphorylation of glucose 6-phosphate Hydrolysis of glucose 6-phosphate by glucose 6-phosphatase by passes the irreversible hexokinase reaction and provides an energetically favorable pathway for the formation of free glucose . Glucose 6-phosphatase , like pyruvate carboxylase , occurs in liver and kidney , but not in muscle . Thus , muscle cannot provide blood glucose by glyconeogenesis . II- Glyconeogenesis from glycerol : Glycerol is produced from fat in adipose tissue . Glycerol is then transported , via blood , to liver and kidney where it is transferred to glycerol 3-p by glycerokinase enzyme . Glycerol 3-p is then oxidized to dihydroxyacetone-p by glycerol 3-p dehydrogenase and NAD+ . Then by the reversal of glycolysis it is transferred to glucose . III- Gluconeogenesis from amino acids : Glucogenic amino acids , by transamination ( TA ) or deamination ( DA ) , can be transferred to pyruvate or other intermediates of citric acid cycle so can be transferred to glucose e.g : Alanine , Cystine , Serine TA or DA Pyruvate . Aspartate TA Oxaloacetate Glutamine TA α-ketpglutarate TCA oxaloacetate N.B Acetyl CoA and compounds that give rise to acetyl CoA ( for example . fatty acids , acetoacetate and ketogenic amino acids ) cannot give rise to a net synthesis of glucose . This is due to irreversible nature of the pyruvate dehydrogenase reaction , which converts pyruvate to 26 acetyl CoA . These compounds give rise instead to ketone bodies and are therefore termed “ketogenic” . VI- Gluconeogenesis from propionate : Fatty acids with odd number of carbon atoms ( obtained from vegetables in diet ) are oxidized in animal tissue to propionate ( 3-carbon acid ) propionate is a minor precursor of glucose in humans but it is a major source of glucose in ruminants . it is converted to glucose as seen below : Metabolism of propionate Cori cycle Lactate is released into the blood by cells that lack mitochondria , such as red blood cells , and by exercising skeletal muscle . In the Cori cycle , blood-borne glucose is converted by exercising muscle tp lactate , which diffuses into the blood . This lactate is taken up by liver and converted to glucose , which is released back into the circulation . Thus the four KEY ENZYMES of gluconeogenesis are : 1- Pyruvate carboxylase . 2- PEP carboxylase . 3- fructose 1.6bisphosphate . 4- G 6-phosphatase . REGULATION OG GLUCONEOGENESIS I- Hormonal regulation : The moment –to- moment regulation of gluconeogenesis is determined primarily by circulating level of hirmones as glucagon , epinephrine , cortisol , insulin , and by the availability of gluconeogenic substrates . In addition , slow adaptive changes in the amount of enzyme activity result from an alternation in the rate of enzyme or degradation , or both . A) Glucagon : This pancreatic islet hormone stimulates gluconeogenesis by two mechanisms : 1.Glucagon ( stimulated by hypoglycemia ) lowers the level of fructose 2,6-bisphosphate , resulting in activation of fructose 1,6-bisphosphate and inhibition of phosphofructokinase-1 . N.B. Fructose 2,6-bisphosphate is synthesized stimulates fructose 6-p by the enzyme PFK-2 Fructose 2,6-bisphosphate stimulates PFK-1( thus stimulates glycolysis ) and inhibits fructose 1,6-bisphosphase ( thus inhibits gluconeogenesis ) . Thus glucagon stimulates gluconeogenesis and inhibitd glycolysis . 2. Glucagon , via an elevation in cAMP level and cAMP-dependent protein kinase activity , stimulates the conversion of pyruvate kinase to its 27 inactive ( phosphorylated ) form . This decreases the conversion of PEP to pyruvate , which has the effect of diverting PEP to the synthesis of glucose . B) Insulin : inhibits gluconeogenesis by decreasing the rate of transcription of the genes coding for the 4 key enzymes of gluconeogenesis ( pyruvate carboxylase , PEP carboxykinase , F 1,6bisphosphatase , G 6-phosphatase ) . C) Cortisol , epinephrine and glucagon ( also diabetes and starvation ) cause induction of the key enzymes of gluconeogenesis . II- ALLOSTERIC REGULATION : Allosteric activation of hepatic pyruvate carboxylase by acetyl CoA occurs during starvation . As result of excessive lipolysis in adipose tissue , the liver is flooded with fatty acids . The rate of formation of acetyl CoA by **-oxidation of these fatty acids exceeds the capacity of the liver to oxidize it to Co2 and H2o . As a result , acetyl CoA accumulates and lead to activation of pyruvate carboxylase . III- Substrate availability : The availability of gluconeogenic precursors , particularly glucogenic amino acids , marked influences the rate of hepatic glucose synthesis . Decreased levels of insulin favor mobilization of amino acids from muscle protein and provide the carbon skeletons for gluconeogenesis . Energy required for gluconeogenesis : During the gluconeogenesis reactions , 6 moles of high-energy bond are utilized : 1. Two moles of pyruvate are required for the synthesis of 1 mole of glucose . As 2 moles of pyruvate are carboxylated by pyruvate carboxylase , 2 moles of ATP are required . 2. Two moles of GTP ( the equivalent of 2 moles of ATP ) are required to convert 2 moles of oxaloacetate to form 2 moles of PEP . 3. An additional 2 moles of ATP are used when 2 moles of phosphoglycerate are phosphorylated , forming 2 moles of 1,3biphosphoglycerate . 4. Energy in the form of reducing equivalents ( NADH ) is required for the conversion of 1,3-biphosphoglycerate to glyceraldehyde 3phosphate . 28 Under fasting condition the energy required for gluconeogenesis is obtained from **-oxidation of fatty acids . Metabolism of Fructose and Galactose Although many monosaccharides have been identified in nature , only a few sugar appears as metabolic intermediates or as structural components in mammals . Glucose is the most common monosaccharides consumed by humans , and its metabolism has been discussed extensively . However , two other monosaccharides , fructose and galactose , occur in significant amounts in the diet and make important contribution to energy metabolism . Galactose is an important component of cell structural carbohydrates . I. FRUCTOSE METABOLISM Dietary sources of fructose About 15% to 20% of the calories contained in the western diet are supplied by fructose ( approximately 100 g/day ) . the major source of fructose is the disaccharide sucrose , which , when cleaved , releases equimolar amounts of fructose and glucose . Fructose is also found as a free monosaccharides in many fruits and vegetables and in honey . Entry of fructose into cells is not insulin dependent ( unlike that of glucose into certain tissues ) . [ Note : In contrast to glucose , fructose is also an extremely poor elicitor of insulin secretion . ] Phosphorylation of fructose For fructose to enter the pathways of intermediary metabolism , it must first be phosphorylated . This can be accomplished by either hexokinase or fructokinase ( see figure ) . Hexokinase phosphorylates glucose in all cells of the body and several additional hexoses can serve as a substrate for this enzyme . However , it has a low affinity ( that is , high K, ) for fructose . Fructokinase provides the primary mechanism for fructose phosphorylation . It is found in the liver ( which processes most of the dietary fructose ) , Kidney , and the small intestine , and converts fructose to fructose 1-phosphate using ATP as the phosphate donor > Fructokinase activity is insulin independent ( while glucokinase is insulin dependent ) thus fructose can be metabolized independent on insulin . This shows the advantage of fructose over glucose in the diet of diabetic patients ( insulin deficiency ) . Cleavage of fructose 1-phosphate 29 Fructose 1-phosphate is not converted to fructose 1,6-bisphosphate as is fructose 6-phosphate , but is cleaved by aldolase B into dihydroxyacetone phosphate ( DHAP ) and D-glyceraldehyde . DHAP can directly enter glycolysis or gluconeogenesis whereas glyceraldehyde can be metabolized by transferring to glyceraldehyde 3-phosphat by a triokinase then enter glycolysis . ( Aldolase A primarily cleaves fructose 1,6-bisphosphate produced during glycolysis to DHAP and glyceraldehyde 3-phosphate ) . High fructose ( sucrose ) in diet increases liver lipogenesis : The rate of fructose metabolism is more rapid than that of glucose because the trioses formed from fructose 1-phosphate by pass phosphofructokinase , the major rat-limiting step in glycolysis . Elevated levels of dietary fructose significantly elevate the rate of lipogenesis in the liver , owing to the rapid production of acety; CoA . Conversion of glucose to fructose by way of sorbitol 1- Synthesis of sorbitol : Aldolase reductase reduces glucose to produce sorbitol ( glucitol ) . it is found in many tissues such as the lens , retina , kidney , placenta , red blood cells , and in cells of the ovaries and seminal vesicles . In the liver , ovaries , sperm , and seminal vesicle cells there is a second enzyme , sorbitol dehydrogenase , that can oxidize the sorbitol to produce fructose . The two-reaction pathway from glucose to fructose in the seminal vesicles is for sperm cells , for which fructose is a preferred carbohydrate energy source . The pathway from sorbitol to fructose in the liver provides a mechanism by which dietary sorbitol is converted into a substrate that can enter glycolysis or gluconeogenesis . 2- The effect of hyperglycemia on sorbitol metabolism : Because insulin is not required for entry of glucose into the cells losted above , large amounts of glucose may enter these cells during times of hyperglycemia , for example , in uncontrolled diabetes . Elevated intracellular glucose concentration , and an adequate supply of NADPH , cause aldose reductase to produce a significant increase in the amount of sorbitol , which , unlike glucose , cannot pass efficiently through cell membranes and therefore remains trapped inside the cell . As result , sorbitol accumulates in these cells , causing strong osmotic effects and therefore cell swelling due to water retention . 30 II. GALACTOSE METABOLISM The major dietary source of galactose is lactose ( galactosyl β-1-4glucose ) . obtained from milk and milk products . Lactose is digested by **-galactosidase ( Lactase ) of the intestinal mucosal cell membrane . Loke fructose , entry of galactose into cells is not insulin dependent . Phosphorylation of galactose Most tissues have a specific enzyme enter for this purpose , galactokinase which produces galactose 1-phosphate . ATP is the phosphate donor . Formation of UDP –galactose Galactose 1-phosphate cannot enter the glyclytic pathway unless it is first converted to UDP –galactose . This occurs in an exchange reaction , in which UDP –glucose reacts with galactose 1-phosphate , producing UDP –galactose and glucose 1-phosphate by the enzyme galactose 1-phosphate uridyltransferase . This enzyme is missing in individuals with classical galactosemia . This leads to accumulation of galactose 1-phosphate and therefore , galactose in cells . The accumulated galactose is shunted into side pathways such as that of galacitol production catalyzed by the same enzyme , aldose reductase that converts glucose to sorbitol . The accumulated galacitol in eye , nerve tissue and liver leads to catract , sever mental retardation and liver damage . Non-classical galactosemia is due to deficiency of galatokinase . Treatment : involves removal of galactose ( and consequently lactose ) from the diet , and the diseased infants are kept on lactose free milk formula . Use of UDP –galactose as a carbon source for glycolysis or gluconeogenesis In order for UDP –galactose to enter the mainstream of glucose metabolism , it must first be converted to its C-4 epimer , UDP –glucose , by 4-epimers . This “new” UDP –glucose can then participate in many biosynthetic reactions , as well as being utilized in the uridyltransferase reaction described above , converting another galactose 1-phosphate into UDP –galactose , and releasing glucose 1-phosphate . Role of UDP –galactose in biosynthetic reactions UDP –galactose can serve as the donor of galactose units in a number of synthetic pathways , including synthesis of : 31 a- Lactose . b- Glycoproteins . c- Glycolipids . d- Glycosaminoglycans . Note : If galactose is not provided by the diet , all tissues requirements for UDP –galactose can be met by the action of 4 –epimerase on UDP – glucose which is efficiently produced from glucose 1-phosphate . Functions of Uronic pathways : Formation of UDP –Glucuronic acid involved in : a- Formation of mucopolysaccharides e.g. ***** sulfate . b- Conjugation with compounds e.g. bilirubin and steroid hormones . c- In most mammals , other than human , it can be transferred to aerobic acid ( vit C. ) BLOOD GLUCOSE Under normal condition , glucose is the only sugar present in blood . In man , glucose is equally distibuted between red cells and plasma . It is freely diffusible into Extracellular fluids . Concentration of blood Glucose : 1- Fasting blood glucose : ( FBG ) : FBG is measured after an overnight fast ( at least 8-10 hours ) . It normal value is 70-100 mg/dl ( 3.89-5.83 mmol/l ) . 2- After a carbohydrate meal , blood glucose reaches to 120-140 mg/dl within a period of ½ to one hour . 3- The blood glucose then begins to decrease returning to fasting level after 2 hours . Regulation and maintenance of Blood Glucose levels ( BGL ) : The BGL at any given time is determined by the balance between 1) The amount of glucose entering the blood stream , and 2) The amount leaving it . Some tissues of the body , such as brain and red blood cells , depend on glucose for energy . Most other tissues require glucose for synthetic reactions , e.g. ribose moiety of nucleotides or the carbohydrate portion of glucoproteins . Therefore , in order to survive , humans must have mechanisms for maintaining BGL . 1) After a meal containing carboyhydrates , blood glucose levels rise . Some of the glucose from the diet is stored in the liver as glycogen ( 5% ) or is converted to fat ( 30-40% ) . The remainder is metabolized in muscle and other tissues e.g. for energy production . 2) After 2 or 3 hours of fasting , this glycogen begins to be degraded by the process of glycogenolysis , and glucose is released into the blood . 32 3) As glycogen stores decrease , adipose triacylglycerate ( fat ) are also degraded , providing fatty acids as an alternative fuel and glycerol for the synthesis of glucose by gluconeogenesis . Amino acids are also released from muscle to serve as gluconeogenesis precursor . 4) During an overnight fast , blood glucose levels are maintained by both glycogenolysis and glyconeogenesis . However after 18-30 hours fasting , liver glycogen stores are completely depleted and gluconeogenesis is the only source of blood glucose . 5) Blood glucose levels are maintained not only during fasting , but also during exercise when muscle cells take up glucose from the blood and oxidize it for energy . During exercise , the liver supplies glucose to the blood by process of glycogenolysis and gluconogenesis . 6) Muscle , although it stores glycogen , does not contribute glucose to the blood , because of the absence of glucose 6-phosphatase in muscle. Thus , the liver plays an important role in the regulation of blood glucose , because it functions both in the removal of glucose from the blood , and in the addition of glucose to the blood . The activity of liver in maintaining normal blood glucose is influenced by various hormones : ROLE OF HORMONES IN THE REGULATION OF BLOOD GLUCOSE : Insulin : is the principal hormone affecting blood glucose levels , and an understanding of its actions is an important prerequisite to the study of diabetes mellitus . Insulin is small protein synthesized in the beta cells of the islels of langerhans of the pancreas . It acts through membrane receptors and its main target tissues are liver , muscle and adipose tissue . The overall effect of insulin is to promote cellular uptake and sstorage of metabolic fuels and these actions are shown in the next figure . 1. Insulin increases the uptake of glucose in muscle and adipose tissue . In contrast , there is no effect of insulin on glucose penetration of liver cells . However , insulin activates the enzymes of glycolysis and glycogenesis in the liver . 2. It activates hexokinase . 3. Insulin stimulates glycogenesis , and lipogenesis . 4. It inhibits hepatic production of glucose ( glucogenolysis ) . Insulin , therefore , promotes the reduction of blood glucose by increasing the rate of glucose utilization And decreasing the rate of delivery of glucose to blood by the liver . 33 Glucagon : The hormone of the **cells of pancreas . Its secretion is stimulated by hypoglycemia . It increases the blood glucose through the following action : 1, glucagon accelerates glycigenolysis in the liver by activating phosphorylase . It has no effect on muscle glycogen . 1. Glucagon stimulates gluconeogenesis from amino acids and lactate . Epinephrine : is secreted by the adrenal medulla as a result of stressful stimuli ( hypoglycemia , fear , excitement , hypoxia , etc ) and leads to glycogenolysis in liver and muscle owing to stimulation of phosphorylase. 1. In muscle , the end product of glyconeogenolysis is lactate , because glucose 6-phosphatase is absent . 2. In liver , glucose is the main product of glycogenolysis , leading to increase in blood glucose . Glucocorticoids : ( as cortisol and cortisone ) are secreted by adrenal cortex . 1. They increase gluconeogenolysis . This is a result of increased protein catabolism in tissues , increased hepatic uptake o amino acids and increased activity of transaminases and other enzymes concerned with gluconeogenesis in the liver . 2. Tissues . Therefore , glucocorticoids are antagonistic to insulin . The renal Glucose Threshold ( RGT ) : When the blood sugar rises to high levels , the kidney exerts a regulatory effect . 1. Normally , glucose is filtered by the glomeruli , but it is reabsorbed and retured to the blood from the tubules . The tubular reabsorption of glucose is affected by phosphorylation using ATP . 2. Since phosphorylation is enzymatic , therefore , the capacity of the tubular system to reabsorb glucose is limited by its content of the responsible enzymes . 3. When the blood glucose level is elevated , the glomerular filtrate contains more glucose than the tubular capacity to handle , and it passes into the urine producing glycosuria . 4. In normal individuals , glycosuria occurs when the level of blood glucos exceeds 170-180 mg/dl , . This is termed the renal Threshold for glucose . Glycosuria may be produced in experimental animal with phlohizin , which inhibits glucose reabsorption in the tubules . The presence of glucosuria is frequently an indication of diabetes mellitus . 34 Types of Glycosuria : Normal urine cntains practically no glucose , Glycosuria is the excretion of glucose in urine . Thgere are different types of glycosuria : A) Hyperglycemia glycosuria : This is due to an increase in blood glucose concentration above the renal threshold level i.e. , 180 mg/dl . Therefore any thing which causes elevation of the blood glucose can cause glycosuria e.g. 1. Diabetes mellitus : which is the most frequent cause of this type . 2. Increased secretion of epinephrine , during periods of excessive pain and emotional excetement ( fear , anxiety , anger ) . 3. Alimentary glycosuria : when large amounts of carbohydrates are absorbed in the gastrointestinal tract more rapidly than can be assimilated . In this case the blood glucose rises above the renal threshold . B) Renal glycosuria : 1. It is not associated with hyperglycemia and occurs even when blood glucose levels are normal . It is caused by defect in the renal tubules , which may be inherited and thus , it is known as “bengin glycosuria” or “diabetes innocence” . 2. Kidney disease e.g. , nephritis . In this case , the tubular capacity to reabsorb glucose is below the rate of golmerular filtration . N.B. : Diabetes Insipidus : It characterized by severe polyuria ( so wrongly diagnosed as DM ) . The volume of urine may reach 30 liter/day . This results from the deficiency of antidiuretic hormone ( ADH ) also known as arginine vasopressin ( AVP ) responsible for water reabsorption from renal tubules of kidney . The urine is highly diluted and this accampanied by drinking of large amounts of water ( polydispia ) . DIABETES MELLITUS ( DM ) Diabetes mellitus is the commonest endocrine disorder encountered in clinical practice . It may be defined as a syndrome characterized by hyperglycemia due to an absolute or relative lack of insulin and/or insulin resistance . The most obvious symptom of diabetes . hyberglycemia , is caused by indequate uptake of glucose from the blood . Because the kidney’s capacity to reabsorb glucose is limited , excessive amounts of blood glucose ( <180 mg/dl or –11 mmol/1 ) results in glucosuria ( glucose in urine ) . High urine glucose concentration produces an osmotic diuresis and therefore polyuria . 35 Hyperosmolarity due to water loss causes polydispia ( thirst felling ) , Since glucose utilization is inhibited , there is an increase in appetite and food consumption ( polyphagia ) . The metabolic disturbances in Diabetes mellitus : The partial or absolute lack of insulin may lead to : 1- Disturbances in Carbohydrate Metabolism : a- Decreased uptake of glucose by adipose tissue and muscles . b- Inhibition of glucose oxidation as result of inhibition of the key glycolytic enzymes . c- Increased liver gluconeogenesis due to : i- Activation of the key enzymes of gluconeogenesis . ii- Increased protein catabolism increased circulating amino acids which provide the fuel for gluconeogenesis . d- Increased liver gluconeogenolysis and decreased glycogenesis . 2- Disturbance in Lipid Membrane : a- Decreased lipogenesis & increased lipolysis in adipose tissue increased circulating free fatty acids ( FFA ) in blood . b- FFA livertriglycerides blood hypertriglyceridemia . c- Decreased activity of lipoprotein lipase elevated levels of lipoprotein fractions reach in triglycerides ( chylomicrons & VLDL ) . d- Excess FFA in liver oxidation to acetyl CoA that accumulates ( due to decreased oxaloacetate synthesized from glucose via pyruvate ) excessive formation of ketone bodies ( acetone , acetoacetic acid , and hydroxybutyric ) decrease PH of blood ( Ketoacidase ) Ketonuria ( ketone bodies in urine ) . 3- Disturbances in protein Metabolism ************************** decreased protein synthesis , and increased gluconeogenesis from amino acids . This results in : a- Wasting and weakening of muscles and decrease in body weight . b- Decreasing resistance to infection . c- Delayed wound healing . Diabetes mellitus is generally subclassified into : a) Insulin dependent iabetes mellitus ( IDDM ) . b) Non-insulin dependent diabetes mellitus ( NIDDM ) . 1- Insulin dependent diabetes mellitus ( IDDM ) or juvenile onset diabetes 36 IDDM accounts for approximately 15% of all diabetes . It can occur at any age but is most common in the young , with a peak incidence between 9 and 14 years of age . The absolute lack of insulin is a consequence of the autoimmune destruction of insulin-producing beta cells . There may be an environmental precipitating factor such as a viral infection . The presence of islets cell antibodies in serum predicts future development of diabetes , It now appears that **-cells destruction is caused by an inflammatory process that occurs over several years & the symptoms are not obvious until all insulin –producing capacity is destroyed . Destruction of **-cells is initiated by the binding of an atibody to a cell surface antigen . The most serious acute symptom of IDDM is ketoacidosis . The odour of acetone on patient’s breath is a characteristic of ketoacidosis . Elevated concentrations of ketones in the blood ( ketosis ) and a lowered blood PH along with hyperglycemia cause excessive water loss . ketoacidosis & dehydration , it left untreated , can lead to coma & death . IDDM patients are treated with injections of insulin obtained from animals or from recombinant DNA technology . II- Non insulin dependent diabetes mellitus ( NIDDM ) NIDDM ACCOUNTS FOR 85 % OF all diabetes and can occur at any age . It is most common between 40 and 80 years . In this condition there is resistance of peripheral tissues to the actions of insulin , so that the insulin level may be normal or even high . The most common cause of insulin resistance is the down-regulation or defect of insulin receptors . Obesity is the most commonly associated clinical feature . Approximately 85% of NIDDM are obeses . Obesity promotes tissue intensitivity to insulin . When the failure of NIDDM to control hyperglycemia is accampanied by other serious medical conditions ( e.g. renal insufficiency , myocardial infraction , or infection ) , a serious metabolic state referred to as Hyperosmolar hyperglycemia non ketosis can result . [ The level of insulin is sufficient to prevent ketosis but does not prevent hyperglycemia and osmotic diuresis ] . The high blood glucose level accompanied by severe dehydration in life-threatening . Treatment of NIDDM usually consists of diet control & exercise . Often , obese patients become more sensitive to insulin when they lose weight . Because sustained muscular activity increases the uptake of glucose without requiring insulin , exercise also decreases hyperglycemia . In some cases , oral hypoglycemia drugs are used . 37 The contrasting features of IDDM and NIDDM are shown in the following table : COMPARISON OF TWO TYPES OF DIABETES MELLITUS Insulin-dependent diabetes mellitus (IDDM) Synonym Age of onsel Prevalence Genetic predisposition Defect or deficiency Ketosis Plasma insulin Acute complications Oral hypoglycemia drugs . Treatment with insulin Non-insulin-dependent diabetes mellitus (NIDDM) Type I ; juvenile-onsel Type II ; adult-onsel diabetes diabetes Usually dueing childhood Frequently after age 35 . or puberly 10%-20% of diagnosed 80%-90% of dignosed diabetics . diabetes . Moderate Very strong **-cells destroyed , Inability of **-cells to eliminating production of produce appropriate insulin . quantities of insulin ; insulin resistance . Common Rare Low to absent Normal to high Ketoacidosis Hyperosmolar coma Unresponsive Responsive Always necessary Usually not required DIAGNOSIS OF DIABETES MELLITUS : DM should not be digested unless hih plasma glucose concentrations have been found in at least two different occasions . The patient is said to be diabetic if he has a fasting venous plasma glucose concentration <140 mg/dl ( 7.8 mmol/l ) or < 200 mg/dl ( 11.1 mmol/l ) two hours afte cho meal or after the oral ingestion of 75 g of glucose , even the fasting concentration is normal . Blood samples may be taken according to any of the following ways : i- Random blood glucose ( RBG ) : RBG is the only test required in an emergency . An RBG of less than 140 mg/dl ( 8mmol/l ) should be expected in non-diabetics . RBG higher than 200 mg/dl ( 11 mmol/l ) usually indicates diabetes mellitus . 2- Fasting blood glucose ( FBG ) : FBG is measured after an overnight fast ( at least 10 hours ) . An FBG is better than RBG for diagnostic purposes . In non-diabetes it is usually lower than 6 mmol/l . fasting values of 6-8 mmol/l should be interpreted as borderline . FBG equal to or above 8 mmol/l on two occasions in diagnostic for diabetes mellitus . 3- Postprandial : 2 hours after a mixed meal ( 120-140 mg/dl ) . 38 4- Oral glucose tolerance test ( OGTT ) : Classically , the diagnosis of diabetes is made on the basis of a patient’s response to an oral glucose load . A baseline bloodsample is first taken after an overnight fast . The patient is then given 75 g of glucose orally , in about 300 ml of water , to be drunk within 5 minutes . Plasma glucose levels are measured every 30 minutes for 2 hours . Urine may also be tested for glucose at time 0 and after 2 hours . The patient should be sitting comfortably throughout the test , should not smoke or exercise , no alcohol or drugs and should have been on a normal diet for at least 3 days prior to the test . Normal and diabetic responses o an oral glucose load are shown in the next figure : Indications Many OGTTS are performed unnecessarily . There are relatively few indications for the test . These include : 1- Borderline fasting or postprandial blood glucose . 2- Presistant glucsouria . 3- Glucsouria in pregnant women . 4- Pregnant women with a family history of diabetes mellitus and those who previously had large babies or unexplained fetal loss . 39 PROTEIN METABOLISM Proteins are organic nitrogenous compounds formed of alpha amino acids. Most of the nitrogen in the diet is consumed in the form of protein, typically amounting 70 to 100 g/ day. Digestion of protein Protein are too large to be absorbed by the intestine and therefore must be hydrolyzed to yield their basic unit amino acids which can be absorbed. The proteolytic enzymes responsible for protein digestion are produced by three different organs: stomach, pancrease and small intestine 1. In the mouth No protein digestion. 2. In the stomach: The digestion of the proteins started in the stomach, which secretes gastric juice containing HCl and the proenzyme pepsinogen APepsins: There are 3 types of pepsin having the same function: Pepsin I, II and III * Pepsins are secreted in the form of pro-enzymes (inactive form).They are called: pepsinogens. Pepsinogen contain extra amino acids in their sequence which prevent them from being catalytically active * Pepsinogens are firstly activated by gastric HCl into pepsin. Then pepsin itself activates pepsinogen ( autoactivation) HCl Pepsinogen----------------------------Pepsin Pepsinogen---------------------------- Pepsin * Action of pepsins They are endopeptidases i.e. act on the amino acids in the middle of the polypeptide chain, hydrolysing the bonds between aromatic amino acids : phenylalanine, tyrosine and tryptophan. So, the products of pepsins digestion are polypeptidechains of variable sizes:Protein-------------------Proteoses--------------Peptone---------------Polypeptides. BRennin (chymosin, Rennet) Activated by calcium ions at pH 4 Action of rennin 40 Rennin acts on the casein, the main milk protein, converting it in the presence of calcium ions into insoluble calcium caseinate (milk clot). Rennin Ca++ Casein---------------------Paracasein------------------Insoluble paracaseinate. calcium (milk clot). Then digestion of calcium paracaseinate is completed by pepsin enzyme. Rennin is important for infants, as the formation of milk clot prevents the rapid passage of milk from the stomach. This gives a sense of fullness. Rennin is absent in the stomach of adults. 3. In the small intestine On entering the small intestine, large polypeptide produced in the stomach by the action of pepsin are further cleaved to oligopeptide and amino acid A. Pancreatic Secretions: (pH:8) 1Trypsin and chymotrypsin Enteropeptidase formerly called trypsin. It is secreted in the form of inactive precursor which is called trypsinogen. Trypsinogen is converted to trypsin first by enterokinase (an enzyme synthesized by and present on the luminal surface of the intestinal mucosal cells of the brush boarder membrane) by removal of hexapeptide from the NH2-terminus of trypsinogen, then trypsin itself activates trypsinogen (autoactivation) Trypsinogen Enterokinase Trypsinogen------------------------------Trypsin Trypsinogen------------------------------Trypsin Chymotrypsinogen------------------Chymotrypsin Procarboxypeptidase-------------carboxypeptidase. * Action of trypsin 1. It is an endopeptidase hydrolyzing the bonds between basic amino acids as arginine and lysine. So, the products of trypsin digestion are polypeptide chains Protein------Proteoses------Peptone---------Polypeptid. 2Trypsin acts as an activator for chymotrypsinogen and procarboxypeptidase converting them into chymotrypsin and carboxypeptidase. 41 * Action of chymotrypsin : It acts on peptide bonds of uncharged amino acids as aromatic amino acids. 2Carboxypeptidase * It is an exopeptidase i.e. acts on the periphery of polypeptide chains produced by the action of endopeptidases. It acts on the peptide bond at the free-COOH of the polypeptide chain liberating single amino acid. 3Collagenase It is an enzyme that catalyzes the, hydrolysis of collagen. B. Intestinal Secretion 1. Aminopeptidase It is an exopeptidase, acting on the peptide bond at the free -NH2 of the polypeptide chain liberating single amino acid. Hormones that stimulate gastrointestinal secretion 1. Gastrin * This hormone is produced by gastric cells. Secretion of this hormone is stimulated by the presence of protein in the stomach. It stimulates pepsin secretion and the release of intrinsic factor from gastric mucosa. 2. Cholecystokinin - pancreozymin ( CCK-PZ ): * This hormone is produced by the mucosa of small intestine. Secretion of this hormone is stimulated by the presence of protein, fat and their digestion products in the intestine. It stimulates the secretion of pancreatic enzymes and contraction of the gall bladder. 3.Secretin * This hormone is produced by the duodenurn and jejunum. Secretion of this hormone is stimulated by the low pH in the duodenum. It stimulates the secretion of pancreatic bicarbonate (HC03 ) and helps the action of CCK-PZ hormone. 42 Digesti0n of Nucleoproteins Nucleoproteins are digested by a group of enzymes produced by intestinal mucosa hydrase, nuclease, phosphatase and nucleosidase. Hydrase Nucleoproteins--------------------------------------- Proteins + Nucleic acid Nuclease Nucleic acid -----------------------------------------Nucleotides Phosphatase Nucleotides------------------------------------------Nucleosides + Phosphate Nucleosidase Nucleosides -------------------------------------------------Nitrogenous base+ Sugar ABSORPTION Under normal conditions the dietary proteins are almost completely digested into amino acids, which are then rapidly absorbed from the intestine into the portal blood. Site of Absorption : Jejunum and ileum Mechanism of amino acids absorption 1. Active transport L-Amino acids are actively absorbed i.e. energy is required, which is derived from sodium pump. They are absorbed by specific carrier protein present in small intestine by mechanism similar to that of glucose absorption i.e. the carrier has a site for an amino acid and another site for sodium There are 5 or more different amino acid carrier systems, each of which can transport a group of closely related amino acids. These groups are 1Small neutral amino acids. 2Large neutral amino acids. 3Basic amino acids. 4Acidic amino acids. 5Imino acids. There is a competition for absorption between the amino acids of each 43 group i.e. amino acid fed in excess can retard the absorption of other amino acids of the same group. 44 Pyridoxal (vit. B6) and Mn++ play a role in amino acids absorption. * There is another hypothesis for the mechanism of transport of amino acids into the cells in which glutathione is shared. Clutathione acts as a carrier for amino acids. absorption of amino acids by this mechanism utilizes 3 ATP molecules. * PLASMA AMINO ACIDS Fed state (after meal) * After the ingestion of a protein rich meal, the amino acids are absorbed from the intestine via the portal circulation to the liver. The branched chain amino acids account for about 20% of the total amino acids present in dietary protein and absorbed to the liver. * Amino acids are then released from the liver to the systemic circulation causing an increase in the. plasma amino acids level. The released branched amino acids account for about 60% of the total amino acids entering the systemic circulation. This means that liver utilizes most of the non branched amino acids. * Muscles extract. the amino acids (mainly the branched chain amino acids) where they undergo 1-Oxidation for energy production 2-Transamination with pyruvate to give alanine, Alanine then may be released to be converted to glucose in liver. CIRCADIAN CHANGES The plasma level of most amino acids does not remain constant throughout 24 hours day. It varies from 4 to 8 mg/100 ml plasma. This depends upon the nutritional state whether it is post-absorption or fed state. This is called a circadian changes of amino acids. Postabsorptive level i.e. 12 hours after last meal. The plasma amino acids tend to be decreased. Amino acids are released from endogenous protein stores into plasma. These stores are mainly muscles and kidneys Muscle : alanine and glutamine account for more than 50% of the total amino acids released from muscle tissue, valine is also released kidney is the major source of release of serine. In addition, the kidney release small, but significant amount of alanine. The released amino acids are taken up 45 Valine is taken up mainly by the brain, where it is utilized. Glutamine is taken up by: gut and kidney: In the gut it is converted into glutamate or free ammonia. In the kidney glutamine may give rise to ammonia. Serine: is taken up by the liver. Alanine: is taken up by the liver. It is the main glucogenic amino acid i.e. amino acid that can be converted to glucose.. Fate of Absorbed Amino Acids The absorbed amino acids may undergo one of the following fates: 1. Protein biosynthesis: The amino acids are incorporated into proteins as plasma proteins, tissue proteins, enzymes and polypeptide hormones. 2. Small peptide synthesis As synthesis of the glutathione tripeptide. 3. Synthesis of specialized products Each amino acid can synthesize certain substances in the body e.g. phenyl alanine gives epinephrine, tryptophan gives serotonine. 4. Excretion of amino acids as urea Conversion of amino acids into urea includes the following processes a-Transamination bDeamination C-Reactions of urea cycle. Excretion of Amino Acids as Urea The first step in the catabolism of amino acids involves the removal of α– amino acids. Once removed, this nitrogen can be incorporated into other compound and excreted This occurs by:I. Removal of -NH2 group as NH3 (ammonia) by: Transamination - Deamination ( Oxidative or Non-Oxidative) - Transamination followed by deamination II. Transport of the produced ammonia. III. Urea formation (by reactions of urea cycle) I. Removal of –NH2 group 1. Transamination 46 The first step in the catabolism of most amino acids is the transfer of their –amino group to –ketoglutarate. The products are –keto acid derived from the original –amino acids and glutamate. This transfer is catalyzed by family of enzymes called aminotransferase. Mechanism Transamination is catalyzed by enzymes termed transaminase or aminotransferase and Pyridoxal phosphate (vitamin-B6) is the coenzyme of the transaminases enzymes. All amino acids may undergo transamination except lysine, therionine, proline and hydroxy proline All transamination reactions are reversible so during amino acid catabolism, this enzyme functions in the direction of glutamate synthesis. Thus glutamate acts as collector of nitrogen from amino acids. Among all transaminases :- Two are present in most mammalian tissues and they are of clinical importance Glutamate transaminase and alanine transaminase. 1Alanine transaminase This enzyme catalyzes the transfer of amino groups from -aminoacids to pyruvate to form alanine-e.g. --ketoglutarate+ Alanine ----------------------- Glutamate + Pyruvate This transaminase has 4 names 1. Alanine transaminase. or 2. Alanine pyruvic transaminase. or 3. Alanine aminotransferase. or 4. Glutamate puryvic transaminase (GPT) 2- Glutamate transaminase : This enzyme catalyzes the transfer of amino group from most amino acids to --ketoglutarate to form glutamate: e.g. -ketoglutarate+ Aspartate------------------------------Glutamate +Oxalacetate This transaminase has also 4 names 1. Glutamate transaminase or 2. Glutamate -ketoglutarate transaminase or 3. Glutamate amino transferase or 4. Glutamate oxaloacetate transaminase (GOT). GOT and GPT are intracellular enzymes. Their level in blood is increased 47 in Myocardial infarction (ischemic heart disease) mostly GOT. Acute hepatitis (liver infection) mostly GPT. Role of pyridoxal phosphate in transamination Pyridoxal phosphate which is covalently linked to the έ–amino group of a specific lysine residue at the active site of the enzyme. Aminotransferases act as an intermediate carrier of the amino group Glutamic acid + Pyrodoxal Phosphate--------------ketoglutarate+ Pyrodoxamine Then Pyrodoxamine +Pyruvic-------------------------Pyrodoxal Phosphate + Alanine Equilibrium of transamination reaction:- For most transamination reactions, the equilibrium constant is near 1, allowing the reaction to function in both directions: amino acid degradation through removal of -amino group ( After high protein diet) and biosynthesis through addition of amino group to -keto acids ( when amino acids are required) 2- Deamination Definition It is. the removal of amino group from an amino acid. Site : The liver and the kidney. Non Oxidative Deamination The enzymes of this mechanism catalyze the removal of the amino groups of the hydroxy amino acids serine, homoserine and therionine. e.g. Oxidative Deamination In contrast to transamination reactions. L-Amino acids which are present in the proteins utilized by human are deaminated by 2 mechanisms:1. All L-amino acids (except glutamate) are deaminated by L-amino acid oxidase. This enzyme is present in a minimal amount in the liver and kidney, thus this mechanism is of little value. The coenzyme is FMN L-amino acid Oxidase Water Amino acid------------------------------------Imino acid--------------------Keto acid NH3 48 2. Glutamate is deaminated by the enzyme : L-glutamate dehydrogenase, which is a highly active enzyme present in most mammalian tissues. NAD or NADP is the co-enzyme L-Glutamate Dehydrogenas Glutamate----------------------------------------------Ketoglutarate + NH3 NADP NADPH+H The amino groups of most amino acids are funneled to -ketoglutarate by means of transamination. Glutamate is unique in that, as it is the only amino acids that undergo rapid oxidative deamination. Therefore, sequential action of transamination ( collection of amino group from amino acids to -ketoglutarate forming glutamate) and subsequent oxidative deamination of that glutamate provide pathway whereby most amino acids can be released as ammonia. Functions of L-glutamate dehydrogenase enzyme 1Removal of -NH2 group as ammonia(NH3) of most amino acids Removal of-NH2 group from most amino acids to form NH3 which is then converted to urea is not done by direct deamination of amino acids, but through aTransamination with -ketoglutarate to form glutamate. bThe product glutamate then undergoes deamination by glutamate dehydrogenase to produce NH3 2Formation of non essential amino acids The reaction catalyzed by glutamate dehydrogenase is reversible i.e. ketoglutarate can be reaminated by free NH2 to form glutamate. Then glutamate can be also transaminated with any -ketoacid to form the corresponding amino acid. Regulation of L-glutamate dehydrogenase * ATP, GTP- and NADH+H+ allosterically inhibits glutamate dehydro-genase.glutamate * ADP allosterically stimulates glutamate dehydrogenase Sources and Transport of Ammonia Sources of ammonia 1-Deamination of amino acids by different tissues. 2- Ammonia produced by the action of the intestinal bacteria on a- Dietary protein. bUrea present in fluids secreted into the gastrointestinal tract. 49 This ammonia is absorbed to the liver via the portal veins. In the liver it is converted to urea. Thus portal blood contains high levels of ammonia than systemic blood. -Kidneys produce ammonia by renal tubular cells in cases of acidosis a mechanism for reg B1ood Ammonia Blood contains traces of ammonia ( 10 – 20 µg/l00 ml ) because the ammonia which is constantly produced in the tissues is rapidly removed from the circulation by the liver. Fate of Ammonia 1- Formation of glutamate Glutamate Dehydrogenase NH3 + -ketoglutarate----------------------------------Glutamate + ketoglutarate Transamination ------------------------------------------Non-essential amino acid 2Glutamine formation Glutamine synthetase is a mitochondrial enzyme present in the kidney and the brain. In the kidney Glutamine is stored in tubular cells. Deamination of glutamine is the main source of NH3 in kidney. This deamination reaction is catalyzed by glutaminase enzyme. Ammonia produced shares in the mechanism for regulation of acid-base balance. In the brain Glutamine formation is the major mechanism for removal of ammonia in the brain but this must be proceeded by the synthesis of glutamate. This is because the supply of blood glutamate is inadequate in the presence of high levels of blood ammonia. -ketoglutarate is an intermediate of citric acid cycle, thus in high levels of blood ammonia, this intermediate will be depleted in the brain Inhibition of citric acid cycle No energy in the brain ammonia intoxication. 50 3Urea formation Urea is the major disposal form of amino group derived from amino acids and account for about 90% of nitrogen containing compound of the urine. Therefore, it is the main end product of protein (amino acids) metabolism. Site : Liver Blood urea : 20 - 40 mg/l00 ml. Blood urea increased in 1) High dietary protein. 2) Kidney disease : failure to excrete urea. 3) Gastro-intestinal haemorrhage as in peptic ulcer Blood is a favorable medium for the growth and multiplication of bacteria in the intestine. Action of intestinal bacteria on proteins (plasma proteins + globin of Hb leads to excessive NH3 production-------------------------- excessive urea production by the liver more than capacity of the kidney to remove. This leads to increase of urea in the blood. Blood urea decreased in: 1) In liver diseases. Steps This occurs by a series of reactions called urea Krebs cycle NH3 Carbamoyl phosphate synthetases +--------------------------------------------------------Carbamoyl Phosphate CO2 2 ATP 2 ADP Ornithine---------------------Citrulline Aspartate ----------------------------Arginosuccinate Arginine------Urea 51 Fumarate --------------------------- 52 One nitrogen of the urea is supplied by free NH3 and the other nitrogen by aspartate. Glutamate is the intermediate precursor of both ammonia through oxidative deamination ( Glutamate dehydrogenase) and aspartate nitrogen through transamination of oxalacetate by aspartate 53 aminotransferase. The carbon and oxygen are supplied by CO2 54 Reactions of the urea cycle The first two reactions leading to the synthesis of urea occur in the mitochondria, whereas remaining cycle enzymes are located in the cytosol . Glutamate dehydrogenase occurs in the mitochondria, providing NH3 for incorporation into carbamoyl phosphate 1. Formation of carbamoyl phosphate Ammonia incorporated into carbamoyl phosphate is provided by oxidative deamination of glutamate. In addition, carbamoyl phosphate synthetase 1 requires N-acetylglutamate and two molecule of ATP for the activity. 2. Formation of citrulline 55 Ornithine is regenerated with each turn of the urea cycle is reacted with carbamoyl phosphate forming citrulline. The release of high energy phosphate of carbamoyl phosphate drives the reactions in the forward direction.. Citrulline is transported to the cytosol. 3. Synthesis of argininosuccinate Citrulline condenses with aspartate to form argininosuccinate. The – amino group of aspartate provide the second nitrogen of the urea. This reaction is driven by the cleavage of ATP to AMP and PP. This is the third and final molecule of ATP consumed in urea formation. 4. Cleavage of the argininosuccinate Argininosuccinate is cleaved to yield arginine and fumarate. Arginine serves as precursor of urea. Fumarate is hydrated to malate and transported to mitochondria re-enters tricarboxylic acid cycle or cytosolic malate can be oxidized to oxalacetate which can be converted to aspartate or glucose. 5. Cleavage of arginine Arginase cleaves arginine to ornithine and urea. Arginase enzyme occurs exclusively in the liver. Thus, other tissues can synthesize arginine, can not produce urea, only liver can cleaves arginine, thereby synthesize urea. Carbamoyl phosphate synthetases:1. Carbamoyl phosphate synthetase I Present in the mitochondria of the liver cells. Use ammonia NH 3 for the formation of carbamoyl phosphate. It is activated by N-acetylglutamate and Mg++ ions and Biotin are the coenzyme which carry CO2. 2 molecules of ATP are used in this reaction. 2. Carbamoyl phosphate synthetase II Present in the cytoplasm of the cells of all tissues. Use glutamine as a source of NH2 for the formation of carbamoyl phosphate. This enzyme is used in pyrimidine biosynthesis. Regulation of urea cycle: N-acetyl glutamate is an essential activator for carbamoyl phosphate synthetase 1 which is the rate limiting step in urea cycle. It is synthesized by acetyl CoA + glutamate. The ammonia produced from glutamic acid and hence from all amino acids by glutamate dehydrogenase is used by carbamoyl phosphate synthetase to form urea Transamination Glut. Dehydro Carbamoyl P Amino acids-----------------------Glutamate----------------NH3----------------Urea The intrahepatic concentrations of N-acetyl glutamate increases after 56 ingestion of high protein diet. Carbamoyl phosphate synthetase 1 acts with mitochondrial glutamate dehydrogenase. Fate of urea. Urea diffuses from the liver and is transported in the blood to the kidney where it is filtered and excreted in the urine. In patients with kidney failure, plasma urea levels are increased Therefore, promoting a greater transfer of urea from the blood to gut. The intestinal urease action on the urea becomes important source of NH3 contributing to hyperammonia seen in this patients. Sources of ammonia Ammonia is produced from metabolism of a variety of compounds. Amino acids are quantitatively the most important source of ammonia 1. Amino acids : most tissues especially liver form ammonia from amino acids by aminotransferase and glutamate dehydrogenase 2. From Glutamine The kidney forms ammonia from glutamine by the action of renal glutaminase. Most of these ammonia are excreted as ammonium 3. From Purine and pyrimidine Catabolism of purine and pyrimidine give rise ammonia as the amino group attached are released as ammonia 4. From bacterial action in the intestine Ammonia is formed by the bacterial degradation of urea in the lumen of the intestine Causes of increased serum ammonia: 1. Acquired hyperammonia Cirrhosis of the liver caused by alcoholism, hepatitis or biliary tract obstruction. Therefore NH3 can not be converted to urea by the diseased liver cells. 2. Hereditory Hyperammonia Result from deficiency of one of 5 enzymes of urea cycle especially carbamoyl phosphate synthetase and ornithin transcarbamoylase. In each case the failure to synthesize urea leads to hyperammonia during the first week following birth Mechanism of ammonia intoxication The mechanism of ammonia toxicity may result from shift in the equilibrium of glutamate dehydrogenase reaction High blood ammonia will deplete -ketoglutarate in the brain Inhibition of citric acid cycle No energy in the brain Ammonia intoxication. Symptoms of ammonia intoxication 1Flapping tremors. 57 2Slurring of speech. 3Blurring of vision. 4Vomiting in infancy. 5Coma. Relation between tricarboxylic acid cycle and urea cycle 1. CO2 needed for urea formation is mainly produced in the course of tricarboxylic acid cycle. 2. Aspartic acid can give oxaloacetate and vice versa (Transamination). Oxaloacetate is the key of Krebs cycle. 3. Fumaric acid produced in urea cycle can be oxidized in tricarboxylic acid cycle. 4. ATP needed for urea cycle are derived from tricarboxylic acid cycle. Fate of -Ketoacids The -ketoacids (the carbon skeleton) remaining after the removal of NH2 group by transamination or deamination of amino acids may be 1Reaminated by NH3 to form .again amino acids (glutamate dehydrogenase). 2Converted either to carbohydrate intermediates. (13 amino acids) or fat intermediates (2 amino acid) or both (5 amino acids.). Those which are converted to carbohydrates are called glycogenic amino acids. Those which are converted to fat are called ketogentic amino acids. Glycogenic Clycogenic&ketogenic Alanine Arginine Aspartic Phenyl alanine Cysteine Tryptophan Glutamic Tyrosine Glycine Histidine Hydroxyproline Methionine Proline Serine Threonine Valine . Ketogenic Leucine Lysine Isoleucine Lysine Amino acids: Metabolism of carbon skeleton The catabolism of the 20 amino acids found in the proteins involves the removal of –amino group followed by breakdown of the resulting carbon skeleton. 58 The catabolism of the carbon skeleton converges to form seven products: oxalacetate, -ketoglutarate, pyruvate, fumarate, acetyl CoA, acetoacetyl CoA and succinyl CoA. These products enter in the pathways of intermediary metabolism, resulting either synthesis of glucose or lipids or in the production of energy through their oxidation to CO2 and H2O by citric acid cycle. Glucogenic and ketogenic amino acids:Amino acids can be classified as glucogenc or ketogenic according to nature of their metabolic end products 1) Ketogenic:- Amino acids whose catabolism yields either actoacetate or one of its precursor acetyl CoA or acetoacetyl CoA. ( Acetone, Acetoacetate and β-hydroxybutyrate). Leucine and lysine are the only 59 exclusively ketogenic amino acid 2) Glucogenic amino acids: Amino acids whose catabolism yields pyruvate or one of the intermediate citric acid cycle. These substrates are substrate in gluconeogenesis and therefore can give rise to glycogen in liver and muscle. A. Amino acids that form oxalacetate Asparagine is hydrolyzed by asparaginase, liberating ammonia and aspartate. By transamination aspartate loses its -amino group forming oxalacetate B. Amino acids that form -ketoglutarate 1. Glutamine is converted to glutamate and ammonia by glutaminase enzyme. Glutamate is converted to -ketoglutarate by transamination or by oxidative deamination by glutamate dehydrogenase 2. Arginine is cleaved by arginase to produce ornithine and urea (final step in urea cycle). Ornithine subsequently undergoes transamination to yield glutamate γ-semialdhyde which is converted to -ketoglutarate 3. Proline is oxidized to –pyrroline 5-carboxylate which is then oxidized to glutamate. Glutamate is converted to -ketoglutarate by transamination or by oxidative deamination by glutamate dehydrogenase 3. Histidine is deaminated and hydrolysed to form N-formiminoglutamate which donates its formimino group to tetrahydrofolate leaving glutamate. Deficiency of folic acid lead to excretion of increased amount of N-forminino glutamate C. Amino acid that form pyruvate 1. Alanine loses its aminogroup by transamination 2. Serine: can be converted to pyruvate by serine dehydratase 3. Glycine can either be converted to serine by addition of a methylene group from N5, N10 methylenetetrahydrofolate or oxidized to CO2 and NH4 4. Cysteine is reduced to cysteine using NADPH+H which can undergo desulfuration to yield pyruvate 5. Threonine can be converted either to pyruvate or -ketobutyrate 60 61 D. Amino acids that form fumarate 1. Phenylalanine and tyrosine. The first step in the catabolism of phenylalanine is hydroxylation forming tyrosine. Then phenylalanine and tyrosine merge leading to fumarate and acetoacetate. Therefore phenylalanine and tyrosine are glucogenic and ketogenic. E. Amino acids form succinyl CoA Degradation of methionine, valine, isoleucine and threonine results in the production of succinyl CoA (TCA cycle intermediate ) 1. Methionine: this sulfur-containing aminoacid is converted to 5adenosylmethionine (SAM) major methyl group donor Synthesis of SAM: Methionine condenses with ATP forming 62 SAM which is high energy containing compound Activated methyl group: the methyl group attached to the tertiary sulfur in SAM is activated and can be transfer to a variety of acceptor molecule e.g methylguanidoacetic acid and converted to Sadenosylhomocysteine Hydrolysis of S-adenosylhomocysteine: after donation of methyl group S-adenosylhomocysteine is hydrolyzed into homocysteine and adenosine Homocysteine can combine with serine, forming cystathionine which is hydrolyzed into -ketobutyrate and cysteine. This interaction has the net effect of converting serine into cysteine 2. Valine and isoleucine are branched chain amino acids that yields succinyl CoA 3. Threonine is dehydrated into -ketobutyrate which is oxidatively decarboxylated into propionyl CoA and Succinyl CoA 63 F. Amino acids that form acetyl CoA, AcetoacetylCoA The following four amino acids form acetyl CoA or acetoacetylCoa directly with -out pyruvate serving as an intermediate 1. Leucine: It is exclusively ketogenic in its catabolism forming acetyl CoA and acetoacetylCoA 2. Isoleucine: It is both ketogenic and glucogenic because its metabolite yields acety CoA and propionyl CoA 3. Lysine : an exclusively ketogenic amino acids. It is unusual in that, neither of its amino group can undergoes transamination as the first step in the catabolism 4. Tryptophan: It is converted to acetoacetyl CoA 5. Phenylalanine and tyrosine Catabolism of the branched chain amino acid The branched chain amino acids isoleucine, leucine and valine are 64 essential amino acids. They are metabolized primarily by peripheral tissues (Muscle) rather than by the liver A. Transamination Removal of the amino groups of all three amino acids is catalyzed by single enzyme branched chain -amino acid aminotransferase B. Oxidative deamination Removal of the carboxyl group from -ketoacid derived from leucine, isoleucine and valine is catalyzed by single enzyme complex, branchedchain-ketoacid dehydrogenase. An inherited deficiency of branched-chain -ketoacid dehydrogenase results in accumulation of the branched chain -ketoacid substrate in urine. Their sweet odour prompted the name maple-syrup urine disease C. Dehydrogenation Oxidation of the acyl CoA products yields either -β unsaturated acyl CoA derivative The catabolism of isoleucine yields acetyl CoA and succinyl CoA rendering it both ketogenic and glucogenic amino acids Valine yields succinyl CoA Leucine yields acetoacetate 65 Biosynthesis of non essential amino acid Non essential amino acid can be synthesized in sufficient amounts from the intermediates of metabolism or from essential amino acids as in tyrosine A. Synthesis from -ketoacid Alanine, aspartate and glutamate are synthesized by transfer of an aminogroup to -ketoacid, pyruvate, oxalacetate and -ketoglutarate respectively Glutamate is unusual in that it can also be synthesized by the reverse of the oxidative deamination by glutamate dehydrogenase B. Synthesis by amidation Glutamine contains an amide linkage with ammonia at carboxyl group. It is formed from glutamate by glutamine synthetase. This reaction is driven by hydrolysis of ATP ATP ADP -ketoglutarate----------glutamate-------------------------Glutamine NH3 This reaction can be considered as major mechanism for detoxification of NH3 in brain and liver 66 Asparagine: this amino acid contains an amide linkage with NH3 at βcarboxyl. It is formed from aspartate by asparagines synthetase C. Tyrosine Tyrosine is formed from phenylalanine by phenylalanine hydroxylase. The reaction requires molecular oxygen and the co-enzyme tetrahydrobiopterin. During the reaction, tetrahydrobiopterin is oxidized to dihydrobiopterin. Tetrahydrobiopterin is regenerated from dihydrobiopterin in separate reaction requiring NADPH D. Proline Glutamate is converted to proline by the intermediate glutamate γ– semialdhyde which cyclize to form –pyrroline 5 caboxylate by reduction yields proline E. Serine, glycine and cysteine Serine arises from 3-phosphoglycerate which is first oxidize to 3phosphopyruvate and then transaminated to 3-phosphoserine. Serine is formed by hydrolysis of phosphoester. It is also formed from glycine by transfer of hydroxymethyl group Glycine It is synthesized from serine by removal of hydroxymethyl group Cysteine: It is synthesized by two consecutive reactions in which homocysteine combine with serine forming cysthionine which in turn hydrolyzed to α–ketobutyrate and cysteine Conversion of Amino Acids to Specialized Products In addition to serving as building blocks for proteins, amino acids are precursors of many nitrogen containing compound that have important physiological functions I. Glycine Glycine is a glycogenic and non essential amino acid. In the body, it may be enter in one of the following pathways: 1. Synthesis of purine bases: Carbon 4, carbon 5 and N 7 are derived from glycine. 2Synthesis of glutathione Glutathione is a tripeptide. Formed of 3 amino acids: glycine, cystein and glutamic It is sometimes called : glutamyl , cysteinyl glycine Steps of synthesis 2 ATP molecules are utilized , one for each peptide bond formed. 67 Forms of glutathione Reduced form G-SH Oxidized form G-S-S-G G-S-S-G is converted to G-SH by the enzyme glutathion reductase and NADPH produced in the first step of HMP shunt. * Functions of glutathione aProtection of cell membranes against oxidizing agent e.g. glutathione protects RBCs from haemolysis bGlutathione acts as a component of an amino acid transport system. e.g. it has a role in absorption of amino acids. cIt acts as an activator for certain enzymes. dGlutathione inactivates insulin hormone in the presence of insulinglutathione transhydrogenase 3Synthesis of creatine : Creatine is a methylguanidoacetic acid. It is formed from 3 amino acids : glycine , arginine and methionine. 68 * Creatine is formed in both the kidney (transamidation),and the liver (transamethylation) to be stored in the muscles in the form of creatine phosphate. Creatine phosphate is high energy compound used in muscle contraction. 69 Creatine phosphokinase CPK CK This is an enzyme which catalyzes the conversion of creatine to creatine phosphate Serum CPK level increases in muscle and heart diseases. 4- Bile salts formation Glycine is conjugated to cholic acid to form glycholic acid (one of bile acids). 5Synthesis of haem Haem is the pigment which together with globin form the haemoglobin These reactions occur in the mitochondria. Then AmLev passes to cytosol to give haem. 2 molecules of ( AmLev ) Propobilinogen Haem. AmLev synthetase is an enzyme present in the mitochondria of liver cell. It is the key enzyme in the synthesis of haem. Pyridoxal phosphate PO4) is the co-enzyme for decarboxylation and also to activate glycine. 6Synthesis of serine The tetrahydroxy folic acid (FH4) acts as a one carbon atom donnor 7- Synthesis of glyoxylic acid Glycine may be converted to glyoxylic acid by aDeamination of glycine 70 bTransamination with α-ketoglutarate The glyoxylic acid either reaminated again to glycine or:oxidized to formic acid. Failure of these 2 reactions leads to the oxidation of glyoxylic acid to oxalate. Excretion of excess amounts of oxalate in the urine is called primary hyperoxaluria. It may leads to the formation of calcium oxalate stone. 8Detoxication reactions Glycine is used in detoxication. It is conjugated in the liver with benzoic acid (which is toxic) giving hippuric acid which is excreted in the urine. Glycine catabolism Breakdown of glycine is mainly done by the action of the enzyme glycine cleavage system. II. Phenyl Alanine and Tyrosine Phenyl alanine is essential amino acid. Tyrosine is non essential amino acid because it is formed in the body from phenyl alanine. Both amino acids are glycogenic and ketogenic amino acids. Phenyl alanine may enter in one of the following metabolic pathways 1Protien biosynthesis. 2Tyrosine formation Phenyl alanine is converted to tyrosine by the enzyme phenyl alanine hydroxylase Tyrosine may give the following compounds 1Epinephrine and nor epinephrine. 2. Melanine pigments. 3. Thyroxine. 71 1* a. bcd* abcde- Epinephrine and nor epinephrine These are 2 hormones secreted by adrenal medulla. They are the most potent stimulant of adrenergic α and β receptors (sympathomimetic). This leads to Increase of heart rate and force of contraction. Vasoconstriction. Relaxation of smooth muscles of bronchi and intestine. Stimulation of glycogenolysis and lipolysis (through cAMP). Epinephrine is used in the treatment of Bronchial asthma Acute allergic diseases. Open angle glaucoma. Heart block. As topical and local vasoconstrictor. * Pheochromocytoma This is a benign tumor affecting the cells of adrenal medulla. The affecting cells secrete abnormal amounts of epinephrine and nor epinephrine. This leads to aHypertension which may be paroxysmal. bAttachs of palpitation , headache , nausea dyspnea , anaxicety pallor and profuse sweeting. Synthesis of epinephrine and nor epinephrine This occurs in adrenal medulla and neurons Tyrosinase DOPA-Decarboxylase Tyrosine-----------------Dihydroxy phenyl alanine (DOPA)----------------------------Oxidase Transferase Dopamine---------------------------Nor-epinephrine---------------------Epinephrine 72 73 2.Melanin pigments These are dark brown to black compounds, derived from tyrosine. They are normally present in melanocytes (pigment cells of skin hair and iris). They give the characterestic colour of skin , hair and iris. Synthesis of melanin This occur in melanocytes Tyrosinase DOPA Oxidase Tyrosine----------------DOPA------------------------DOPA quinone----------Melanine 74 3-Thyroxine This is a hormone that produced by the thyroid gland. Synthesis of thyroxine Synthesis of thyroid hormone begins by iodination of the tyrosine radicals which are present in thyroglobulin protein. This protein is present in the thyroid follicles and contains carbohydrate (glycoprotein). lodinated thyroglobulin is then brokendown releasing T3 and T4. On stimulation of thyroid gland by TSH , T4 and T3 are released into plasma. 75 Within the plasma 99.95 of T3.and T4 are transported inassociation of 2 proteins (thyroine binding proteins) aThyroxine binding gIobulin : It is the major transporter. bThyroxine binding prealbumin About O.O57% of T4 and T3 are present in the free unbound state. Free T3 and T4 are the metabolically active hormones in the plasma. If tyrosine is not needed for the synthesis of epinephrine nor epinephrine ,melanin and.thyroxine , it is oxidized to homogentisic acid fumaric acid and acetoacetic acid. Inborn errors of phenyl alanine and tyrosine metabolism 1Phenylketonuria. 2Tyrosinosis. 3Alkaptonuria. 4Albinism. 1- Phenylketonuria: This is a condition resulting from congenital deficiency of phenyl-alanine hydroxylase. Tyrosine will not be formed. Phenylalnine is increased in the blood and converted to phenyl pyruvic and phenyl lactic which appear in the urine. Effect of phenylketonuria The infants and children affected with phenylketonuria:developmental retardation for unknown reason. Diagnosis The plasma of every newly born infant is routinly tested for elevated phenyl alanine 4 days after birth. Prevention : 76 Children are maintained on a diet containing a very low level of phenyl alanine. This diet can be terminated at 6 years of age , when high concentration of phenyl alanine can not cause injury to the brain. 2-Tyrosinosis: Deficiency of parahydroxy phenyl pyruvic acid oxidase. This results in excretion of large amount of parahydroxy phenyl pyruvic acid in the urine. 77 3-Alkaptonuria: Condition resulting from deficiency of homogentisic acid oxidase. homogentisic acid will accumulate and appear in the urine. When homogentisic acid is voided with the urine it is oxidized in the air to a black pigment. Effect Generalized pigmentation of connective tissue (ochronosis) and arthritis. 4- Albinism: Deficiency of tyrosinase enzyme in melanocytes , so the body can not synthesize melanine pigments. i.e. skin is whitish , hair is also whitish and iris is colourless. III. Tryptophan Tryptophan is an essential amino acid. It is glucogenic and ketogenic amino acid. Tryptophan has the following metabolic pathways 1Serotonin formation Serotonin is a substance present in the brain , platelets , intestine and mast cells. Action of serotonin Vasoconstriction of blood vessels. Contraction of smooth muscle fibers. Stimulatory transmitor in the brain. 78 * Mono amine oxidase(M.A.O.): An enzyme which oxidize serotonin to 5-hydroxy indole acetic acid which passes to the urine. Inhibition of this enzyme by some drugs (e.g. iproniazid) leads to accumulation of serotonin and Psychic stimulation. Serotonin may be converted to a substance called melatonine which is Nacetyl,5 methoxy serotonin; It is found in the tissue of pineal body in the brain. It appears to depress the gonadal function. Melatonine is conjugated with sulphate in the liver then excreted. 2Niacin (nicotinic acid) formation Niacin is a memberof vit. B complex. Every 60 mg of tryptophan are converted to 1 mg niacin. People depending on diet deficient in either tryptophan or niacin (e.g. maize) will be suffered from a disease called pellagra. End product of tryptophan metabolism is indole and skatol which are excreted in the stool giving its characteristic odour. Indole may be absorbed to the liver, hydro -xylated and conjugated to form indican which is excreted in the urine. 79 80 IV. GLUTAMIC ACID 81 It is a glycogenic amino acid and it is non essential amino acid. It is important in transamination. Glutamic acid enters in the formation of 1-Glutathione It is a tripeptide formed of 3 amino acids glycine cysteine and glutamic acid 2-Folic acid It is a member of vit. B-complex formed from glutamic acid, paraaminobenzoic acid and pteridine ring. 3-Gamma amino butyric acid = GABA It is formed from glutamic acid by decarboxylation. GABA is an inhibitory transmitter in the brain and its deficiency leads to convulsions especially in children 4-Glutamine : it is formed by combination of glutamic acid with ammonia. Functions of glutamine A) Glutamine is stored in the kidney and by the action of glutaminase enzyme it gives ammonia and glutamic acid again. Ammonia is used in the regulation of acid-base balance. B) Glutamine is the source of N3 N9 of purines. C) Glutamine formation in the brain removes excess NH3 formed in the brain D) Glutamine is used in detoxication of phenyl acetic acid. V. Sulphur Containing Amino Acids These are Cysteine , cystin and methionine. Methionine is essential amino acid but cysteine and cystin are formed in the body. All are glycogenic amino acids. Cysteine It enters in the formation of 1. Glutathione 82 2. Taurine formed in the liver. Taurine is conjugated to bile acid to form one of the bile salts. 3- Thioethanolamine Thioethanolamine is a constituent of CoASH fatty acid synthetase, mevalonate synthetase and other enzymes related- to lipid metabolism. 4- Cystin Cystin is produced from 2 molecules of cysteine by oxidation. 4. Many hormones as insulin are rich in cysteine. Also , many enzymes contain in their active center -SH group derived from cysteine. 6- H2S and sulphate prbduction -Sulphite oxidase will result in presence of H2S and SO2 in expired air and it causes mental retardation. Methionine It is essential amino acid it is important for a- Entering in structure of protein synthesis. b- Its conversion to S-adenosyl methionine , the main methyl group donner in the body. c.Its conversion to homocysteine and cysteine. 83 Transmethylation It is a transfer of methyl group from a methyl donner to a methyl acceptor. Methyl donner : S-adenosylmethionine the main methyl group donner. Methyl acceptor e.g. Nor-adrenaline CH3 Adrenaline. Uracil CH3 Thymine. Guanidoacetic acid CH3 Creatine. Ethanolaminehydrate CH3 Choline. VII. SERINE Glycogenic , non essential amino acid. It enters in: 1- Ethanolamine and choline base : 2- Sphingosine Sphingosine is formed from serine and palmityl CoA 3- Cysteine It is formed of serine and methionine as follows: 84 4-Phospholipids and phosphoproteins Serine is an important constituent of phosphoprotein e.g. casein and phospholipids e.g. cephaline. DYNAMIC STATE OF BODY PROTEINS All the body proteins with the exception of collagen, are in a constant state of degradation and resynthesis. The total amount of protein synthesized in the body is much greater than that degradated This provides the body needs of protein for 1Growth 2Replacement of cells after their death and 3For milk protein during lactation. PROTEIN REQUIREMENT An adult man needs 1 gm/kg body weight/day and he synthesizes about .400 gram of protein each day. The exact daily protein requirement depends on many factors 1-Type of protein a-Protein of high biological value It contains all essential amino acids and are easily digested. b.Good quality protein It does not contain all essential amino acids. c.Proteins of plants Are deficient of most amino acids and are not easily digested. 2-Age , sex and lactation Children lactating females and women need more protein than adult males do. NITROGEN BALANCE Nitrogen balance means that nitrogen intake is equal to nitrogen loss from the body Nitrogen intake Nitrogen is taken in the form of proteins of diet. Every 100 gm proteins contain 16 gm of nitrogen. Nitrogen loss Nitrogen is lost from the body 85 1- 23- In the urine Urea 20 - 40 gm/day. Uric acid 0.7 gm/day. Hippuric acid 0.7 gm/day. Ammonia 0.7 gm/day. Creatinine 0.7- l.7 gm/day. Creatine 0-0.2 gm/day. In the feces One gram/day is excreted in the feces. In milk and menstrual fluids in female. POSITIVE AND NEGATI\'E NITROGEN BALANCE Positive nitrogen balance It means that nitrogen intake is greater than nitrogen loss. It occurs in conditions where the formation of tissue protein is increased e.g. growing children and muscle training. Negative nitrogen balance: It means that nitrogen intake is less than nitrogen loss. It occurs in conditions where the breakdown of tissue protein is increased e.g. Diabetes mellitus and starvation. FUNCTIONS OF PROTEIN IN THE BODY 1Protein enters in structure of every body cell. 2- All enzymes are protein in nature. 3- Some hormones are protein in nature e.g. pituitary hormones , glucagon and insulin. 4- Some hormones are amino acids derivatives e.g. nor-epinephrine, epinephrine and thyroxine. 5- Nucleic acids (RNA and DNA) are present in the form of nucleoproteins? 6- Plasma proteins have many functions including immunity , blood coagulation and fluid exchange. HYPOPROTEINOSIS This is a condition due to dietary deficiency of proteins. In adult This leads to hypoproteinaemia with subsequent oederna , weakness of muscle , anaemia and increased susceptibility to infections. In infants a- Kwashiorker This is a disease resulting from deficiency of dietary protein only. It leads to growth retardation, anaemia, and anorexia. b- Marsmus This is a disease resulting from deficiency of dietary protein together with 86 dietary carbohydrate and fat. 87 Enumerate 5 of the following 1. Essential amino acid 2. Non polar side chain amino acid 3. Conjugated proteins 4. Amino acids involved in urea cycle 5. Biologically active compound derived from glycine 6. Metabollic errors of aromatic neutral amino acid 88 Lipid Metabolism DIGESTION, ABSORPTION AND TRANSPORT OF LIPIDS An Adult ingests 60 ----150 g of lipid/ day more than 90% of which is triglycerids (TG). The remainder is made up of cholesterol, cholesterol ester, phospholipids and free fatty acids. DIGESTION OF DIETARY FATS: Order of events that happens in digesting fats A-Limited digestion in mouth and stomach Milk lipids can be degraded by the gastric lipase (active at neutral pH), not used in adult but has a role in infant. In adults dietary lipids are not digested to any extent in mouth or the stomach. B. Emulsification of dietary lipids in the small intestine: Physical (not chemical) breakdown of fats. Since the lipids are water insoluble, therefore, enzymatic hydrolysis of lipids can occur only on the surface of the lipids droplet. Mixed Micelles: Emulsification of lipids in the GI-Tract leads to mixed micelles. Mixed Micelles are partially degraded lipids with detergent-like properties. They contain bile acids, dietary lipids and phospholipids, fat-soluble vitamins, cholesterol, etc. Chemical breakdown: Pancreatic Lipolytic enzymes break down triacylglycerols to individual fatty acids. These enzymes are hormonally regulated as follows: -Cholecystokinin (CCK; pancreozymin) is a small peptide hormone secreted from lower duodenum in response to presence of lipids. It causes the gall bladder to contract releasing the bile and digestive enzymes from pancrease. -Secretin: small peptide hormone released from intestine in response to low pH of the chime. It stimulates bicarbonates 89 secretion from pancrease leading to neutral pH required for enzymatic activity. -Co-lipase: is a second protein secreted from pancrease helps to anchor and stabilize the lipase enzyme. 1) Triacylglycerol degradation Lipase Degrades triacylglycerols. The proenzyme (zymogen) is activated by the presence of bile acids, phospholipids, or regulatory enzyme called co-lipase. 90 2)Cholesterol ester degradation Cholesteryl Ester Hydrolase Degrades dietary cholesterol, usually present in the form of cholesteryl esters. The proenzyme is activated by bile salts. 3)Phospholipids degradation Phospholipase A2 Degrades phospholipids. The proenzyme (zymogen) is activated by trypsin and Ca+2 The enzyme can remove fatty acid at carbon 2 of phospholipids leading to lysophospholipids 91 ABSORPTION: Individual fatty acids (not triacylglycerols) free cholesterol and 2-monoacylglycerol are absorbed through the brush border. Resynthesis of triacylglycerol& Cholesterol esters by Intestinal Mucosal Cells: Once inside intestinal enterocytes, triacylglycerols and cholesteryl esters are reformed. 92 Secretion of lipids from intestinal mucosal cells TG and cholesterol are very hydrophobic, must be packaged in a soluble form. This form consists of lipids surrounded by thin layer of proteins, phospholipids and free cholesterol. The newly formed triacylglycerol droplets are then assembled into a chylomicron: CHYLOMICRON ASSEMBLY: Chylomicron is a form of lipoprotein, consisting of lipid and protein. This assembly occurs in the intestinal epithelial cells. Secretion of Chylomicron: it is transport into the lymphatic system for the most part. Chylomicrons always travel in lymph to the extrahepatic tissues -- except short chained fats. Short-chained fats (up to 10 carbons): The majority of these fats are carried through the portal circulation back to the liver -- not the lymph! Here is reason that fetuses utilize short-chains fats -they are absorbed and sent directly to the infant's liver, rather than going through lymphatic system. Long-chained fats: (greater than 12 carbons): The majority of them are carried through the lymphatic system and thereby distributed throughout the body. Fats travel to liver: Fats make their way back to liver, from lymphatic or portal circulation. They then utilize lipoproteins (see below) to get distributed to target tissues. Utilization at target tissue: At the target tissue, fats are broken back down again, using lipoprotein lipase. Lipoprotein Lipase: Triacylglycerol ------> Monoacylglycerol + 2 Fatty Acids. FATE OF ABSORBED LIPIDS A-Uptake by tissues The absorbed chylomicrons are taken up by the tissues after hydrolysis to glycerol and free fatty acids. This requires the 93 enzyme lipoprotein lipase present in the vascular endothelium of extrahepatic tissues particulary in adipose tissues. This enzyme hydrolysis 90% of the TG in chylomicrons leaving remnant chylomicron. B- Utilization by tissues 1-Oxidation: Fatty Acids------------acetyl CoA------Krebs cycle--CO2 + water +energy 2-Conversion to glucose : glycerol-------glycerol phosphate----reverse of glycolysis--------glucose 3- Formation of tissues fat: all tissues can synthesize all their requirement of structural fat C- Storage Adipose tissues take up and stores a large part of the absorbed fat. As a store of energy because fat is better than carbohydrates and proteins in its caloric value and its associated with less water in storage Types of body lipids 1- Tissue lipids: This includes structural and functional lipids. They includes lipids present in cell membrane, mitochondria , lysosomal, and plasma membrane. They consist of phospholipids, glycolipids and cholesterol with little triglycerides. It is generally rich in unsaturated fatty acids. Tissues lipid does not changes in composition or amount with changes in the nutritional state and hence termed constant element 2- Depot Lipids: They are mainly fat TG that is stored in adipose tissues. It is principle reservoir of energy. It is vary according to nutritional state of the individual and hence termed variable element Lipogenesis Lipogenesis is the biosynthesis of triacylglycerols . It occurs in most tissues specially adipose tissue, liver and lactating mammary glands. It is concerned with the storage of excess glucose after a high carbohydrate meal and also provides a large part of milk fat. The biosynthesis of TG requires: 94 Biosynthesis of glycerol phosphate . Biosynthesis of fatty acids . Glycerol + FA's Triglycerides . I- Biosynthesis of glycerol (glycerol-3-phosphate) : Reduction Glycolysis Glucose dihydroxyacetone glycerol-3-p NADHH+ II- Biosynthesis of Fatty acids : Like many other degradative and synthesis processes ( e.g. glycogenolysis and glycogenesis ), fatty acids synthesis (lipogenesis) was formerly considered to be merely the reversal of oxidation within the mitochondria . However, it is now apparent that a highly active extramitochondrial system is responsible for the complete synthesis of palmitate from acetylCoA in the cytosol. Another system for fatty acids chain elongation is also present in the liver endoplasmic reticulum. There are three enzyme systems that are involved in the biosynthesis of fatty acids from acetyl-CoA (resulted from glucose by glycolysis). These include extramitochondrial, mitochondrial, and microsomal systems. 1- Extramitochondrial ( cytosolic ) or De novo systems : This is the main pathway for de novo synthesis of fatty acids in the cytosol. It is present in many tissues including liver, kidney, lung, mammary glands and adipose tissues. It needs the following precursors and cofactors. Acetyl-CoA. NADPH. ATP, Mn2+ HCO3- (as a source of CO2) Biotin (co-carboxylase) . The end product of de novo system is free palmitate (C16) Sources of reduced NADP ( NADPH+H) required for de novo synthesis of FA : Hexose monophosphate shunt (G-6-p dehydrogenase and 6-PG dehydrogenase reactions) . 95 Extramitochondrial isocitrate dehydrogenase reaction: This reaction is the same that occurs in the mitochondria ( in Kreb's cycle ) but use NADP instead of NAD as co-dehydrogenase . Acetyl-CoA is the Principal Building Block of Fatty Acids It is formed from carbohydrate via the oxidation of pyruvate within the mitochondria. However, acetyl CoA does not diffuse readily into the extramitochodrial cytosol, the principal site of fatty acid synthesis. The activity of the extramitochondrial ATP –citratre lyase, like the "malic enzyme" increase in activity in the well-fed state, closely paralleling the activity of the fatty-acid synthesizing system. It is now believed that utilization of glucose for lipogenesis is by way of citrate. The pathway involves glycolysis followed by the oxidative decarboxylation of pyruvate to acetyl-CoA within the mitochondria and subsequent condensation with oxaloacetate to form citrate, as part of the citric acid cycle. This is followed by the translocation of citrate into the extramitochondrial compartment via the transcarboxylate transporter, where in the presence of CoA and ATP ,it undergoes cleavage to acetyl-CoA and oxaloacetate catalyzed by ATP-citrate lyase . The acetyl-CoA is then available for malonyl-CoA formation and synthesis to palmitate. The resulting oxaloacetate can form malate via NADH-linked malate dehydrogenase, followed by the generation of NADPH via the malic enzyme. In turn, the NADPH becomes available for 96 lipogenesis. This pathway is means of transferring reducing equivalents from extramitochondrial NADH to NADP . Alternatively, malate can be transported into the mitochondrion, where it is able to reform oxaloacetate . It is to be noted that the citrate ( tricarboxylate ) transporter in the mitochondrial membrane requires malate to exchange with citrate . Eight molecules of acetyl-CoA are used for the formation of one mole of palmitic acid (C16). Seven of the 8 mole of acetyl-CoA are first converted to malonyl-CoA before being utilized for the enzyme acetyl-CoA carboxylase . The process occurs in 7 repeated cycles, each requires 2 mol of NADPH+H . The overall reaction can be summarized in the following equations : Acetyl-CoA + 7 Malonyl-CoA + 14 NADPH+H Palmitic acid + 8 CoA + 14 NADP+ + 7CO2 + 6H2O Fatty acid synthase multienzyme : An acyl-carrier protein ( ACP ) and the group of enzymes responsible for the biosynthesis of palmitate are all found in one polypeptide chain forming a multienzyme complex (FA synthase). The aggregation of all the enzymes of a particular 97 pathway into one multienzyme functional unit has many advantages: a) It offers great efficiency and freedom from interference by competing reactions. b) The synthesis of all enzymes is a dimer. In mammals, each monomer is identical, consisting of one remarkable polypeptide chain containing 7 enzyme activities of fatty acid synthase and ACP with a 4'–phosphopantetheine-SH group. In close proximity is another thiol of a cysteine residue of 3-ketoacyl synthase (condensing enzyme) of the other monomer (see figure) 98 99 Short and long-term mechanisms regulate lipogenesis Long-chain fatty acid synthesis is controlled in the short term by allosteric and covalent modification of enzymes and in the long term by changes ih gene expression governing sites of synthesis of enzymes. A- Citrate and Acyl-CoA Regulate Acetyl-CoA Carboxylase The most important reaction regulating the lipogenic pathway is at the acetyl-CoA carboxylase step. Acetyl-CoA carboxylase is an allosteric enzyme which is activated by citrate, and increases in concentration in the well-fed state and is an indicator of plentiful supply of acetyl-CoA . However, it is inhibited by long-chain acyl-CoA molecules, an example of metabolic negative feedback inhibition by a product of a reaction sequence. Thus, if acyl-CoA accumulates because it is not esterified quickly enough, it will automatically reduce the synthesis of new fatty acid. Likewise, if acyl-CoA accumulates as a result of increased lipolysis or an influx of free fatty acid into the tissue, this will also inhibit synthesis of new fatty acid. Allosteric activation of the enzyme involves aggregation from monomeric to polymeric configuration of several million in molecular mass. Acyl-CoA may also inhibit the mitochondrial tricarboxylate transporter, thus preventing activation of the enzyme by egress of citrate from the mitochondria into the cytosol. B- Insulin Also Regulates Lipogenesis : Insulin stimulates lipogenesis by several mechanisms : I] It increases the transport of glucose into the cell ( e.g. in adipose tissue ) and thereby increase the availability of both for FA synthesis and glycerol-3- phosphate for esterification of newly formed fatty acids . II] Insulin converts the inactive form of pyruvate dehydrogenase to the active form . III] Insulin activates acetyl CoA carboxylase . 100 C- The Fatty Acid Synthase Complex and Acetyl-CoA carboxylase are adaptive Enzymes : These enzymes adapt to the body's physiological needs by increasing in total amounts in feed and decreasing in fasting, feeding of fat, and in diabetes. Insulin is an important hormone causing gene expression and induction of enzyme biosynthesis, and glucagon antagonize this effect. Feeding fats, containing polyunsaturated fatty acids causes inhibition of expression of key enzymes of glycolysis and lipogenesis. These mechanisms for longer-term regulation of lipogenesis, take several days to become fully manifested and augment the direct an immediate effect of free fatty acids and hormones such as insulin and glucagons. 2- Mitochondrial system for FA synthesis: This system can elongate FA of moderate chain length (C10 or C12) into long chain FA (C16 to C20), but cannot catalyze de novo synthesis of FA. It works only under aerobic condition and its physiologic significance is uncertain. The enzymes used are those of β-oxidation , working in the opposite direction, except for the conversion of enoyl-CoA to the corresponding saturated acyl-CoA which is catalyzed by the enzyme enoyl-CoA reductase using NADPH+H as co-reductase 3- Microsomal system ( elongase ) : This is the main system for elongation of fatty acids from C10 to C22 . Its enzymes convert acyl-CoA to the longer by 2 carbons using malonyl-CoA as acetyl donor and NADPH as hydrogen donor. It cannot catalyze do novo synthesis of FA, and its enzymes can work separately. 101 Desaturation of fatty acids : 1-Synthesis of monosaturated FA : An enzyme, Δ9 desaturase, in the endoplasmic reticulum will catalyze the conversion of palmitoyl-CoA or stearyl-CoA to palmitoleyl-CoA or oleyl-CoA respectively. Oxygen and either NADPH or NADH are necessary for the reaction. 2- Synthesis of polyunsaturated FA : Additional double bonds introduced into the existing monounsaturated FA are always separated by a methylene group. In animals, the additional double bonds are all introduced between the existing double bonds and the carboxyl group (in plants they may be introduced between the existing double bonds and ω-carbon). Since animals are unable to synthesize either linoleic (ω6) or α-linoleinic (ω3 ) acids because the required desaturase is absent , these acids must be supplied in 102 diet to accomplish the synthesis of other members of ω6 and ω3 families of polyunsaturated fatty acids . Linoleic acids may be converted to arachidonic acid. The pathway is first by dehydration of the CoA ester through γ-linolenate , followed by the addition of two-carbon unit via malonyl-CoA in the microsomal system for chain elongation to give eicosatrienoate . The latter forms arachidonic acid by further dehydrogenation. The nutritional requirements for arachidonic acid may thus be dispensed with if there is adequate linoleate in the diet . 103 Oxidation of fatty acid 1- β - oxidation of fatty acids Fatty acids are both oxidized to acetyl-CoA and synthesized from acetyl-CoA . Although the starting material of one process is identical to the product of the other and the chemical stages involved are comparable, fatty acids oxidation is not the simple reverse of fatty acid biosynthesis but an entirely different process taking place in a separate compartment of the cell. The separation of fatty acid oxidation from biosynthesis allows each process to be individually controlled and integrated with tissue requirements. Fatty acid oxidation takes place in mitochondria; each step involves acyl-CoA derivatives catalyzed by separate enzymes, utilizes NAD+ and FAD as coenzymes, and generate ATP. In contrast, fatty acid biosynthesis (lipogenesis) takes place in the cytosol, involves acyl derivatives continuously attached to a multienzyme complex, utilizes NADP+ as coenzyme, and requires both ATP and bicarbonate ion . Fatty acid oxidation is an aerobic, and requiring the presence of oxygen . FA oxidation requires preliminary activation of the FA to acyl-CoA by the enzyme acyl-CoA synthetase ( FA thiokinase ) present in the outer mitochondrial membrane : Acyl-CoA Synthetase Fatty Acid + ATP + CoASH Acyl-CoA + AMP + PPi Role of carnitine in FA transport : Acyl-CoA (synthesized in the outer mitochondrial membrane) cannot penetrate the inner mitochondrial membrane to enter the β-oxidation reaction or to be elongated. Therefore, introduction of the acyl radical into the mitochondrial matrix requires a carrier known as carnitine (β-hydroxy-γtrimethylammonium butyrate) which is widely distributed and is particularly abundant in muscle. It is synthesized from lysine and methionine in liver and kidney. An enzyme, carnitine palmitoyltranseferase I, present in outer mitochondrial 104 membrane, converts long-chain acyl-CoA to acylcarnitine which is able to penetrate the inner membrane of mitochondrial and gain access to β-oxidation system of enzymes. Carnitine acylcarnitine translocase acts as inner membrane carnitine exchange transporter. 105 B- Oxidation of Fatty Acids 106 Energy yield from complete oxidation of one mole of palmitic acid ( C16 ) : The oxidation of one mole of palmitate ( C16 ) results in the formation of 8 moles of acetyl-CoA ( C2 ) by passing through 7 β-oxidation cycles . Each β-oxidation cycle yield 5 mole of ATP (2 moles via FADH2 and 3 moles via NADH+H). Thus we get: 7 β-oxidation cycles x 5 moles of ATP = 35 ATP ( 35 ATP ) . The 8 moles of acetyl-CoA ( from β-oxidation ) each yield 12 ATP via Kreb's cycle thus : 12 x 8 = 96 ATP . Thus the total ATP = 35 + 96 = 131 ATP ( 131 ATP ) . Since 2 ATP were lost in the activation of FA to acyl-CoA , therefore the net gain will be : 131 - 2 = 129 ATP . Biomedical importance Increased fatty acid oxidation is characteristic of starvation and of diabetes mellitus, leading to ketone body production by the liver (ketosis). Ketone bodies are acidic and when produced in excess over long periods, as in diabetes, cause ketoacidosis, which is ultimately fatal. Because gluconeogenesis is dependent upon fatty acid oxidation, any impairment in fatty acid oxidation leads to hypoglycemia. This occurs in various states of carnitine palmitoyltransferase , or inhibition of fatty acid oxidation by poisons , e.g., hypoglycin Regulation of β-oxidation : 107 Regulation of long-chain fatty acids oxidation in liver : It was shown that, livers of starved animals oxidize considerably more fatty acids to CO2 while esterify less to acylglycerol. This may be explained by the fact that carnitine palmitiyltransferase I activity in the outer mitochondrial membrane regulate the entery of long-chain acyl groups into mitochondria prior to β-oxidation. Its activity is low in fed state, when fatty acids oxidation is depressed , and high in starvation, when fatty acid oxidation increases . Malonyl-CoA , the initial intermediate in fatty acids biosynthesis , which increases in concentration in the fed state , inhibits this enzyme , thereby switching off β-oxidation . Thus , in the fed condition there is active lipogenesis and high malonyl-CoA , which inhibits carnitine palmitoyltransferase . Free fatty acids , in the fed state , enter the liver cell in low concentrations and are nearly all esterified to acylglycerols and transported out of the liver in very low density lipoproteins ( VLDL ). However, as the concentrations of free fatty acids increases with onset of starvation, acetyl-CoA carboxylase is inhibited directly by acylCoA , and malonyl-CoA decreases , releasing the inhibition of carnitine palmitoyltransferase I and allowing more acyl-CoA to β-oxidized. These events are reinforced in starvation by decrease in [ insulin ] / [ glucagons ] ratio . 108 Oxidation of odd-carbon fatty acids : II- Omega oxidation of fatty acids : FA's of average chain length, and to lesser extent long chain FA's, may initially undergo ω-oxidation ( oxidation of the terminal methyl group), forming ω-dicarboxylic acid. The enzymes required are present in the liver microcosoma. The dicarboxylic acid may be further shortened by β-oxidation. III- Alpha oxidation : α-oxidation results in the removal of one carbon at a time from the carboxyl end of FA molecule . It occurs in brain tissue. It does not require CoA and does not generate ATP. It is important for the oxidation of FA with methyl groups on βcarbon, which block β-oxidation e.g. Phytanic acid present in animal and milk fats and in certain plants. 109 Biosynthesis of triacylglycerol The enzyme glycerokinase is present only in the liver and kidney but not in the adipose tissues. Thus glycerol-3-phosphate involved in the synthesis of TG can be derived directly from glycerol in the liver and kidney while in adipose tissues it is derived from glucose via glycolytic process. 110 Metabolism of adipose tissue : Triacylglycerols of adipose tissues are continually undergoing : 1] Lipolysis ( hydrolysis ) . 2] Reestrification (lipogenesis) The resultant of these two processes determines the amount of FFA released from adipose tissue into the blood . The glycerol liberated from the process of lipolysis cannot be utilized by the fat cells due to deficiency of glycerokinase , so glycerol liberated diffuse to the blood to be utilized by the liver . 111 The reesterification of the FA requires activation to acylCoA and a supply of glycerol-3-p the later is obtained from glucose via glycolysis . High carbohydrate diet stimulates insulin release which increases the uptake and oxidation of glucose by adipose tissue that results in increased glycerol-3-P formation and hence stimulates the reesterification of FA and decreases their release in blood. During fasting the rate of lipolysis exceeds the rate of reesterification so TG in adipose tissue decreases and FFA's in blood increase. The hydrolysis of TG is controlled by the enzyme hormone sensitive lipase (HSL). This enzyme may exist in 2 forms, an active phosphorylated form " lipase a " and an inactive dephosphorylated form " lipase b ". The phosphate is attached to serine residues in the enzyme protein. The process of activation and inactivation of HSL is controlled by many hormones. Control of adipose tissue lipolysis . ( TSH , thyroid-stimulating hormone ; FFA , free fatty acids ) Note the cascade sequence of reactions affording amplification at each step . The lipolytic stimulus is " switched off " by removal of the stimulating hormone ; the action of lipase phosphatase ; the inhibition of the lipase and adenyl cyclase by high concentrations of FFA : the inhibition of adenyl cyclase by adenosine ; and the removal of cAMP by the action of phosphodiesterase . ACTH , TSH , and glucagons may not activate adenyl cyclase in vivo , since the concentration of each hormone required in vitro is much higher than is found in the circulation . Positive ion ((+)) and negative ((-)) regulatory effects are represented by broken lines and substrate flow by solid lines . 112 HSL is inhibited by insulin, PGE1 and nicotinic acid. It is stimulated by epinephrine, norepinephrine, glucagons, and ACTH. Thyroxin increases the sensitivity of adenylate cyclase to the effect of epinephrine and norepinephrine probably by increasing the membrane receptors. Glucocorticoids increase lipolysis by augmenting the synthesis of HSL enzyme . Brown adipose tissue : It is a type of adipose tissue that differs from white adipose tissue by having a well developed blood supply and a high content of mitochondria and cytochromes but low activity of ATP synthase . It is involved in metabolism particularly at times when heat generation is necessary. Thus, the tissue is extremely active as in some species of animals exposed to cold and in the newborn animal. Though not a prominent tissue in human, it is present in normal individuals, where it appears to be responsible for " diet-induced thermogenesis" which may account for how some persons can eat and not get fat. It is noteworthy that brown adipose tissue is reduced or absent in obese persons. Phospholipids and Glycolipids Metabolism These compounds are present in high concentrations in the brain, egg yolk, liver and kidney. They are not essential dietary components, every tissue is able to synthesize them provided that the essential FA's, choline and inistol are available. 1] Biosynthesis of Phospholipids : 113 Degradation and remodeling of phospholipids : Although phospholipids are actively degraded, each portion of the molecule can turn over at different rate. This is due to the presence of enzyme that allow partial degradation followed by resynthesis. Phospholipase A2 catalyzes the hydrolysis of the ester bond in position 2 to form lysophospholipids which in turn may be reacylated by another acyl-CoA in the presence of 114 acyl transferase. Then lysophospholipids is attacked by lysophospholipase ( phospholipase B ) removing 1-acyl group Phospholipase A1 , attacks the ester bond in position 1 while phospholipase A2 attacks the bond at position 2 of phospholipids . Phospholipase C ( which is the major toxin of some bacteria ) attacks the ester bond in position 3 . Phospholipase D is an enzyme, described mainly in plants , that hydrolyzes the nitrogenous base from phospholipids . Lysolecithin may be formed by an alternative route involves the enzyme lecithin-cholesterolacyltransferase(LCAT). This enzyme , found in plasma and synthesized in liver , catalyzes the transfer of a fatty acids residue from the 2 position of lecithin to cholesterol to form cholesteryl ester and is considered to be responsible for much of cholestryl ester in plasma lipoprotein . Lecithin + Cholesterol LCAT Lysolecithin + Cholesteryl ester 115 Biosynthesis of Sphingolipids 116 Ketone bodies metabolism 1- Ketogenesis : Under certain metabolic conditions associated with a high rate of fatty acids oxidation , the liver produces considerable quantities of acetoacetate and 3-hydroxybutyrate. Acetoacetate continually undergoes spontaneous decarboxylation to yield acetone. These three substances are collectively known as ketone bodies (or acetone bodies). Acetoacetate and 3hydroxybutyrate are interconverted by the mitochondrial enzyme 3-hydroxybutyrate dehydrogenase . 117 Ketogenesis becomes of great physiological importance during starvation when carbohydrate stores are depleted and the oxidation of fats becomes a major source of energy to the body. The brain normally uses glucose as the only fuel. Brain takes some time to adopt to ketone body utilization as an energy sources in starvation but cannot utilize fatty acids . 2- Ketolysis : Ketone bodies formed in liver go via the blood to extrahepatic tissue where they become available to oxidation to CO2 and H2O. Although most tissues can oxidize FAs , they can more easily oxidize ketone bodies. Thus ketogenesis may be considered as a preparatory step performed by the liver to facilitate the oxidation of FA by extrahepatic tissue . 118 While liver is equipped with an active enzymatic mechanism for the production of acetoacetate from acetoacetyl-CoA , acetoacetate once formed cannot be reactivated directly in the liver except in the cytosol , where it is a precursor in cholesterol synthesis, a much less active pathway. This accounts for the net production of ketone bodies by the liver. Ketosis Normally the level of ketone bodies in the blood do not exceed 1 mg/dl. However in some cases the rate of ketogenesis may exceed much the rate of ketolysis leading to increased ketone bodies in the blood " Ketonemia " then excess ketone bodies are excreted in urine " Ketonuria " . This pathological case is called Ketosis , Ketosis occurs in states of decreased carbohydrate utilization e.g : 1] Starvation . 2] Uncontrolled diabetes mellitus . 3] Low carbohydrates and high fat diet . 4] Pregnancy . All the above conditions are associated with decreased insulin relative to the antiinsulin hormone , leading to increased lipolysis and release of FFA from the adipose tissue as well as decreased oxidation of glucose by the liver . This increases the uptake and oxidation of FA by the liver, forming excess acetylCoA. The decreased glucose oxidation decreases the availability of oxaloacetate in the liver mitochondria which leads to inhibition of acetyl-CoA oxidation via kreb's cycle , also decreased the translocation of acetyl-CoA to the cytosol for FA synthesis. This results in the accumulation of acetyl-CoA and increased HMG-CoA production leads to increased ketogenesis . 119 LIPID TRANSPORT AND STORAGE * Plasma Lipoproteins* They are molecular complex of lipids and specific proteins called apolipoproteins General Structure of Lipoproteins: the major lipids carried by lipoproteins particle are triglycerides and cholesterol obtained from diet or denovo synthesis. They are composed of Core of non-polar lipids. Monolayer of phospholipids surrounding the core. Integral and peripheral apoproteins dispersed throughout. Function: They function to keep lipids soluble since they transport them in plasma and to provide an efficient mechanism for delivering their lipids to tissues, 1. Categories of Lipoproteins: Lipoproteins are often categorized according to density, which is dependent upon the relative amount of protein present. The more protein present, the higher the density of the lipoprotein. 120 Chylomicrons -- These are the lowest density, therefore contain the most lipid and lowest percentage of proteins. Largest in size lipoproteins. Half-life about 30 minutes VLDL: Very Low Density. These are the initial lipoproteins to carry triacylglycerols from the liver to target tissues. Half-life about a few hours IDL: Intermediate Density . Transient lipoprotein. VLDL's become IDL's when they lose the triacylglycerol component. LDL: Low density. Half-life is about a few days. IDL's become LDL's when they lose the Apo-E protein. HDL2: High Density lipoprotein. The most dense particle. Half-life = a few days. HDL3: High Density . It is another variety of HDL. Plasma lipoprotein can be separated on the basis of their electrophoretic mobility. 2-Apoproteins -They serve as structural component of the lipoprotein particles -They provide recognition sites for cell-surface receptors -They also serve as activator for enzymes involved in lipoprotein metabolism -They are divided into classes A to H and subclasses apoA-1 and apo C-II TYPES OF APO-PROTEINS: Apo-A1: Found in HDL and Chylomicrons. It activates LCAT, which in turn promotes breakdown of cholesterol-containing lipoproteins. Apo-B100: Found in LDL and VLDL and serves as a receptor for uptake of these particles by target cells. Also plays structural role. Apo-B48: Structural protein in chylomicrons. 121 Apo-CII: Serves as a cofactor for lipoprotein lipase -- to aid in breakdown of lipoproteins. Found in HDL2, VLDL, and Chylomicrons. Apo-E: Serves as a receptor-ligand for HDL particles and Chylomicrons. Also found on ApoE-Rich HDL, a special form of HDL. The presence of different alleles of this protein in brain cells has related to Alzheimer's Disease, in recent research. Metabolism of Chylomicron 1-Chylomicrons are assembled in the intestinal mucosal cells and carry dietary TG, cholesterol, cholesterol ester and other lipids made in these cells. They are released by exocytosis from the intestinal mucosal cells and travel through lymphatic vessels to thoracic duct then to the blood. These particles are called nascent chylomicrons and contain apo-B-48. 2- When nascent chylomicrons reach the plasma they received apo-E and apo-C II from circulating HDL. *-Apo-E together with apo-B48 help to recognize the modified particles by receptors located on the hepatocytes membrane. *-Apo-C II is necessary for the activation of lipoprotein lipase 3- Degradation of TG contained in chylomicron by lipoprotein lipase which is found on the capillary wall of most tissues especially adipose tissues and muscles. This enzyme is activated 122 by apo C II leads to the formation of free fatty acids and monoacylglycerol. Chylomicron becomes smaller in size and more dense. The remaining particles are known as chylomicron remnants. 4- the chylomicron remanants are removed from the circulation by the liver. Hepatocytes membrane contain lipoprotein receptors that recognize the combination of apo-B48 and apo-E. Remnant bind to these receptors and are taken up into hepatocyte cells by endocytosis fused with the lysosomes and degraded into their components, free fatty acids, TG, cholesterol. Metabolism of very low density lipoprotein (VLDL) 1-VLDL are formed in the liver, composed mainly of TG ( endogenously synthesized ) and their function is to carry TG from liver to peripheral tissues. 2- VLDL are released from liver containing apo- B100 and apo A. Once they released into circulation they received apo CII and apo E from circulating HDL 3- In the circulation TG are removed from VLDL by lipoprotein lipase ----- modified VLDL which become smaller in size and more dense. Apo E and apo CII are returned again to HDL 4-Finally, cholesterol; ester are transferred from HDL to VLDL in an exchange reaction with the reverse transfer of TG and phospholipids from VLDL to HDL. 5- After this modification VLDL have been converted to LDL. 123 Metabolism of low density lipoprotein "LDL" - LDL particles retain apo-B-100, but lose their other lipoproteins to HDL. They contain less TG than VLDL and have high concentration of cholesterol and cholesterol ester. -The primary function of LDL is to provide cholesterol to the peripheral tissues. They provide cholesterol to the peripheral cells via receptors on the cell surface membrane that recognize apo-B 100 - LDL receptors are negatively charged glycoproteins that are clustered in pits on the cell membranes. The intracellular side of the pit is coated with protein called clathrin which stabilizes the shape of the pit. LDL are internalized as an intact particles. - The vesicle containing LDL loses its clathrin coat and fuses with other similar vesicles called endosomes -The pH of endosome falls due to proton puming activity of endosomal ATPase causing separation of LDL from its receptors 124 125 - The receptor can be recycled whereas LDL are degraded by lysosomal enzymes into amino acids, cholesterol, fatty acids and phospholipids. -The number of cell surface receptors for LDL varies according to the availability of these LDL particles and need of the cell. Therefore there is down-regulation of the cell receptor if there is large amount of LDL in plasma and Up-regulation if the cells are starved for cholesterol they increase cell surface receptors. Effect of endocytosed cholesterol on cell cholesterol content:-High cholesterol content inhibits HMG-CoA reductase via allosteric site. -High cholesterol inhibit de novo synthesis by inhibiting HMGCoA reductase gene activation -If cholesterol is not required by the cells immediately, it is stored as cholesterol ester by the aid of the enzyme lecithin cholesterol acyltransferase (LCAT) Metabolism of high density lipoprotein "HDL" Why are they good? -It is synthesized in a nasent immature form in the liver and are released into bloodstream by exocytosis, absorbs cholesterol in many extra-hepatic tissues. It is like a cholesterol sponge. -It is secreted in the blood, modified after interaction with chylomicrons and VLDL as it exchanges lipids and apoproteins -HDL has the higher amount of proteins and the lowest amount of TG than other lipoproteins so it is the most dense. -HDL transfers the apoproteins CII and E to chylomicron and VLDL. -HDL also functions in picking up cholesterol from the surface of the cells and from lipoproteins to return it back to the liver so it retards the atherosclerotic process. -Esterification of free cholesterol: once cholesterol is taken up by HDL, it is immediately esterified by Phosphatidylcholinecholesterolacyltransferase PCAT, plasma enzyme synthesized by the liver and activated by Apo A-1 of the HDL. The resulting 126 cholesterol ester is so hydrophobic that is effectively trapped in the HDL and can no longer transfer to cell membrane. -HDL can remove cholesterol ester to VLDL by cholesteroyl ester transfer protein -HDL particle are taken by the liver by receptor mediated endocytosis and the cholesteryl ester are degraded and converted into Bile acids, Recycled with lipoprotein or secreted into bile. ESTROGEN: Estrogen increases the levels of HDL. Hence premenopausal women are at lower rish for CHD than men. Other things (exercise and loss of body fat) also increase HDL. Function of HDL 1- transfer apoproteins to other lipoproteins 2- pick up cholesterol from from cell membrane 3- convert cholesterol into cholesterol ester 4- transport cholesterol ester to other lipoproteins or to liver 127 128 Three cellular receptors involved in lipoprotein metabolism: All of the following receptors recognize various Apo-Proteins and take up lipoprotein-particles into the cell via receptormediated endocytosis. LDL-RECEPTOR -- "Apo-BE Receptor" -- It recognizes ApoBE particles -- i.e. Apo-B100 or Apo-E It recognizes and takes up LDL-Particles; widely distributed throughout different tissues. Regulated -- The delivery of the cholesterol to the cell inhibits this receptor! That is, the product of the endocytosis gives negative feedback. Functions: Regulation of LDL-Levels in blood, redistribution and utilization of cholesterol. Structure -- The Apo-BE Receptor is a single-pass transmembrane protein, which sports the following domains... a defect in can result in Hypercholesterolemia: Ligand-Binding domain: It will bind both Apo-B100 and ApoE. O and N-Linked glycosylation regions. EGF-Homologous domain REMNANT RECEPTOR -- Apo-E Found in the liver only. It recognizes Chylomicron Remnants and Apo-E Rich HDL receptors. (Remnants are the leftovers of the chylomicron particles, after they have already dumped off their fatty acids). Remember -- Chylomicrons remnants still have lots of cholesterol in them! Function: Uptake of cholesterol-loaded remnants and delivery of cholesterol to the liver. NOT REGULATED SCAVENGER RECEPTOR -- Uptake of oxidized or chemically modified LDL-Particles by Monocytes in circulation or Macrophages in tissues. Functions: Degradation and uptake of modified ("damaged") lipoproteins, and uptake of bacteria to protect us from endotoxic shock. 129 Lipoprotein Chylomicrons Very low density lipoproteins (VLDL) Low density lipoproteins (LDL) Main apoproteins Function B48, A-1, C11, E Largest LP, synthesized in gut after a meal . Not presentin normal fasting plasma . Main carrier of dietary TG. B100 C-11, E Generated from VLDL in the circulation . Main carrier of cholesterol . B100 High density A-1, A-11 Lipoproteins (HDL) Apolipoproteins A-I A-H B100 B48 C-I C-II E Synthesized in the liver Main carrier of endogen -ously produced TG. Site of synthesis Intestine , Liver Intestine , Liver Liver Smallest but most abundant. Protective function. Takes chole sterol from extra hepatic tissues to the liver for excretion . Functions Activates LCAT TG & Cholesterol transport Bind to LDL receptor . Intestine TG Transport Liver Activates LCAT Liver Activates LPL Liver , Intestine Binds to LDL receptor and Macrophage . probably also to another specific liver receptor . 130 Cholesterol Metabolism Cholesterol is a typical animal sterol, the brain and egg yolk are very rich sources. The liver, Kidney and meat are also rich sources. Average diet provides about 0.5g/day. Cholesterol is not a dietary essential, the greater part of body cholesterol arise by synthesis. Cholesterol biosynthesis: All tissue can synthesize and degrade most of their cholesterol requirement in situ. The liver is the main source of plasma cholesterol, but the intestine also participates. 131 Estrification of Cholesterol : a- Within the cell : Cholesterol-OH + Acyl-CoA Acyl-cholesterol acyl transferase ACAT Cholesterol ester b- In Plasma : Cholesterol-OH Leuthin-cholesterol transferase + Lecithin acyl(LCAT) Cholesterol ester + Lysolecithin Regulation of cholesterol biosynthesis : HMG-CoA reductase is the key enzyme in the biosynthesis of cholesterol. This enzyme exists in 2 forms a "phosphorylated inactive form" and "dephosphorylated active form" phosphorylation is catalyzed by a specific " reductase kinase " and dephosphorylation by a " protein phosphatase " . HMG-CoA reductase is activated by insulin and inactivated by glucagon, thus the enzyme activity is inhibited during starvation, directly acetyl-CoA to the formation of ketone bodies, and increased by feeding of carbohydrates . 132 Factors influence the cholesterol balance in tissues : At the tissue level, in the following are considered to govern the cholesterol balance of cells. The increase in cholesterol influx in tissues is caused by: 1- Uptake of cholesterol-containing lipoproteins by receptors, e.g., the LDL receptor or the scavenger receptor. 2- Uptake of cholesterol-containing lipoproteins by a nonreceptor-membrane pathway. 3- Uptake of free cholesterol from cholesterol-rich lipoproteins to the cell membrane. 4- Cholesterol synthesis. 5- Hydrolysis of cholesteryl esters by the enzyme cholesterol ester hydrolase . The decrease is caused by : 1- Efflux of cholesterol from the membrane to lipoproteins of low cholesterol potential, particularly to HDL, promoted by LCAT. 2- Esterification of cholesterol by ACAT. 3- Utilization of cholesterol for synthesis of other steroids, such as hormones, or bile acids in liver. Catabolism and excretion of cholesterol : About 1 gram of cholesterol undergoes catabolism and excretion from the body per day, About 50% is excreted in stools in the form of bile acids, the remainder is also excreted in stools in the form of neutral sterols. 1- Neutral sterols : Cholesterol is excreted via the bile into the small intestine, part of the secreted cholesterol is reabsorbed ( enterohepatic circulation ), and part is partially reduced by intestinal bacteria to coprostanol ( coprosterol ) . Coprostanol together with a small amount of cholesterol are excreted in feces. 2- Bile acids : Two primary bile acids are synthesized from cholesterol by liver, namely, cholic acid and chenodeoxycholic acid, 133 principally the former. First cholesterol is converted, by 7 αhydroxylase, to 7 α-hydroxycholesterol, which undergoes oxidative shortening of the side chain forming cholyl-CoA and chenodeoxycholyl-CoA. These are then reacted with glycine (75%) or taurine (25%) to form glycol- or tauracholic ( or chenodeoxycholic) acids. This conjugated bile acids are secreted in bile as their K+ or Na+ salty ( bile salts ) . In small intestine, by the action of intestinal bacteria, part of bile salts undergoes deconjugation and α -dehydroxylation . In this way cholic and chenodeoxycholic acids are converted to deoxycholic and lithocholic acids ( secondary bile acids ) . The greater portion of bile acids are reabsorbed from the ileum into the portal circulation, taken up the liver and reexcreted in the bile (enterohepatic circulation). The bile acids that not reabsorbed are excreted in feces. Bile salts are important for the digestion and absorption of fats because of their ability to lower the surface tension . 134 135 Cholesterol, Atherosclerosis and Cornary heart disease( CHD ) : 1- There is a positive correlation between raised serum lipids, specially cholesterol, and cholesteryl ester and the incidence of CHD and atherosclerosis in humans. 2- Atherosclerosis is characterized by the deposition of cholesterol, cholesterol ester, of lipoproteins VLDL and LDL in connective tissue of arterial walls. 3- Disease in which prolonged elevated levels of LDL and VLDL occur in the blood (such as diabetes mellitus, hypothyroidism and other conditions of hyperlipidemia) are accompanied by premature or severe atherosclerosis. 4- People with high level of LDL have an increased risk of CHD 5- People with high level of HDL have a decreased risk. This is because LDL transports cholesterol to tissue while HDL transports cholesterol from the tissues and arterial walls to liver to be excreted. 6- Additional factors which may play a role in atherosclerosis include hypertension, obesity and lack of exercise. 136 Lipid-lowering drugs Drug group HMG CoA reductase inhibitors Fibrates Nicotinic acid and derivatives Principal actions Inhibit cholesterol biosnthesis and lower total and LDL cholesterol Activate LPL and lower TG and LDL cholesterol Inhibit lipolysis within adipocyte lower TG, increase HDL Effect of diet on serum cholesterol level : The factors play the greatest role in determining individual blood cholesterol concentrations, but the dietary and environmental factors also play a part . 1- Diet rich in cholesterol raises its concentration in the blood and increases the formation of fatty deposits in the arteries. 2- Fats rich in saturated FA's e.g eggs and animal fats, raise blood cholesterol level. 3- Naturally occurring oils that contain a high proportion of polyunsaturated fatty acids include sunflower, cottonseed, corn and soybean oil and olive oil that contain a high concentration of monosaturated lowered plasma cholesterol concentration. The reason for cholesterol-lowering effect of polyunsaturated FA's is still not clear. However several hypotheses have been advanced, including the stimulation of cholesterol excretion into the intestine and the stimulation of the oxidation of cholesterol to bile acids. Diets rich in palmitic acid inhibits the conversion of cholesterol to bile acids. 137 Factor Cholesterol Lipoproteins Blood pressure Weight Exercise Menopause Coffe Smoking Alcohol intake Stress Increased Risk High Raised LDL High Overweight Non or infrequent Post-menopausal High intake Smokers High High Decreased Risk Low Raised HDL Normal Correct weight Moderate or regular Pre-menopausal Low intake Non-smokers Low Role of liver in lipid metabolism : The liver has a central and unique role in lipid metabolism: 1- It facilitates the digestion and absorption of lipids by the production of bile which contains cholesterol and bile salts synthesized within the liver de novo or from uptake of lipoprotein cholesterol. 2- Liver has active enzyme systems for synthesizing, oxidizing and desaturating fatty acids and for synthesizing TG and phospholipids. 3- Liver converts FA's to ketone bodies ( Ketogenesis ) . 4- Liver plays an integral part synthesis of cholesterol and in the synthesis and metabolism of plasma lipoproteins. Fatty liver The lipid content of the normal liver is approximately 4% of which only about 1/4 is TG, for a variety of reasons, lipidsmainly triacylglycerol-can accumulate in the liver. Excessive accumulation is regarded as a pathogenic condition called fatty liver. When accumulation of lipid is chronic, fibrotic changes occur in the cells that progress to cirrhosis and impaired liver function . Fartty liver may result from : 138 1- Over-feeding of Fats : A very high fat diet increases the uptake of fats by the liver. If this exceeds the capacity of the liver to synthesize VLDL, fatty liver occurs. 2- Over-feeding of Carbohydrates : If the excess carbohydrate overload the capacity of the liver to store glycogen, it is converted to TG. If this exceeds the capacity of the liver to synthesize VLDL, fatty liver occurs. 3- Overmobilization of Fat from Depot to Liver : This occurs in conditions characterized by decreased carbohydrate utilization, e.g. diabetes mellitus, starvation and low carbohydrate diet, such conditions are associated with the release of large amount of FFA's from adipose tissue. Increasing amounts of FFA's are taken up by the liver and esterified to TG, which exceeds the capacity of the liver to form VLDL leading to fatty liver which is usually accompanied by hyperlipemia . 4- Inhibition of Fatty acids oxidation : This directs fatty acids to esterification and formation of TG, leading to fatty liver. Fatty acids oxidation is inhibited in the following conditions: a- Deficiency of Pantothenic acid: ↓ CoA formation ↓ acyl-CoA b- Deficiency of carnitine : leads to decrease in the FA transfer into the mitochondria for B-oxidation . c- Alcoholism : Alcoholic fatty liver is chiefly caused by decreased oxidation of FA as a result of oxidation of ethanol to acetaldehyde . Alcohol dehydrogenase CH3CH2OH + NAD+ Ethanol CH3CHO + NADHH+ Acetaldehyde 139 This leads to increased NADH/NAD+ ratio that causes a shift to the left in the equilibrium "malate ↔ oxaloacetate" ( in Kreb's cycle ) leads to inhibition of FA oxidation . In addition acetaldehyde is further oxidized to acetyl-CoA which increase FA synthesis . 5- Undermobilization of Fat from liver to blood : Triacylglycerols synthesized in the liver are normaly mobilized to the blood by forming VLDL. Failure to synthesize VLDL may be caused by either decreased protein synthesis or decreased phospholipids synthesis, leading to fatty liver. a- Decreased protein synthesis : This occurs in the following conditions i- Deficiency of the essential amino acids due to mal-nutrition and intake of diet deficiency in proteins of high biological value ii- Toxic factors , e.g. carbon tetrachloride , chloroform , phosphorus and arsenic , which inhibit hepatic protein synthesis iii- Abetalipoprotein , a genetic defect caused by failure of the liver to synthesize the protein apo-100 . b- Decreased phospholipids synthesis : This occurs in the following conditions : I] Deficiency of essential fatty acids ( EFA ) which are required in the 2-position of phospholipids . Their deficiency may be produced by a diet deficient in plant oils . It may also be produced by increased cholesterol intake since cholesterol competes with phospholipids for estrification with EFA . Ii] Deficiency of choline which is required for biosynthesis of phospholipids . It may be resulted from a diet deficient in choline , in methyl donor ( methionine and betaine ) and in vitamins involved methyl group synthesis ( folic acid and B12 ) A deficiency of Vitamin E enhances the hepatic necrosis of the choline deficiency type of fatty liver . Added Vit E or a source of selenium has a protective effect by combating lipid peroxidation . 140 Iii] Deficiency of inositol which is required for biosynthesis of lipositol . Inositol deficiency may be induced by a diet poor in pyridoxine or rich in biotin . Lipotropic factors : These are factors, which help the metabolization of TG from the liver. Therefore, they include substances essential for the biosynthesis of phospholipids and of proteins, thus helping the formation of VLDL which is needed for mobilization of TG from the liver Lipotropic factors include: 1- EFA . 2- Inositol . 3- Choline . 4- Methionine and betaine . 5- Folic acid , vitB12 , pantothenic acid . EICOSANOIDS Prostaglandins and related compounds : Archidonic acid and some other C20 fatty acids with methylene-interrupted bonds give rise to eicosanoids , physiologically and pharmacologically active compounds known as : 1- Prostaglandins ( PG ) . 2- Thromboxanes ( TX ) . 3- Leukotrienes ( LT ) . 4- Lipoxins ( LX ) . Biosynthesis of Eicosanoids : Archidonate, usually derived from the 2-position of phospholipids in plasma membrane, as a result of phospholipase A2 activity, is the precursor for the synthesis of PGs, TXs , LTs and LXs compounds . Specific agonists stimulate the cells to release arachidonic acid. These agonists have specific target cells, e.g. Thrombin 141 stimulates the arachidonic acid platelets and Bradykinin causes the release of arachidonic acid in renal tubules. Two pathways are involved in eicosanoids biosynthesis: 1- The cyclooxygenase system : which converts arachidonic acid to PG and TX . This cyclooxygenase system is inhibited by NSAID such as aspirin , indometacin , ibuprofen or ketoprofen . 2- The lipooxygenase system which converts arachidonic acid to LT and LX . Regulation of arachidonic acid release : An inhibitory protein ( Lipocortin ), whose synthesis is induced by glucocorticoids, regulates the release of arachidonic acid from membrane phospholipids. This inhibitory effect on arachidonic acid release is the so-called anti-inflammatory action of steroids since PG's and PG-like substances play an important role in inflammatory reactions. Prostaglandins ( PG ) - These are 20-carbons hydroxyl fatty acids containing a 5member ring . - Therapeutic uses : 1- Prevention of conception. 2- Induction of labor at term. 142 3- Termination of pregnancy. 4- Prevention of allevation of gastric ulcer. 5- Control of inflammation. 6- Control of blood pressure. 7- Relief of asthma. 8- Relief of nasal congestion. - PG's increase c-AMP in platelets, thyroid and lung but lower cAMP in adipose. - Prostacyclins ( PGI2 ) are a type of prostaglandins produced by blood vessel walls and are potent inhibitors of platelet aggregation and inhibitors of adherence to the endothelial surface . PGI2 are vasodilator particularly for arteries . Thromboxanes A2 ( TXA2 ) : - TXA2 is a 20-carbon hydroxyl fatty acid conyaining 6-member ring . - It is synthesized in platelets and has opposite effect to PGI2 , contracting arteries promote platelet aggregation . - So TXA2 and PGI2 have antagonist effects . Fish oil and vascular heart disease : The low incidence of heart disease and diminished platelet aggregation in Eskimos may be due to high intake of fish oil which contain eicosapentaenoic acid ( EPA ). This EPA gives a rise to a type of PGI which is PGI3 which is more potent as astiaggregator of platelets than PGI2. Also EPA gives rise to a type of TX which is TX3 is a weaker aggregator than TXA2 . The net result is that the balance of activity is shifted toward no aggregation. 143 Fish oil also lowers plasma cholesterol, TG, LDL and VLDL while it increases HDL concentration, so protect against atherosclerosis. Leukotrienes ( LT ) - Leukotrienes are hydroxyl fatty acid derivatives of arachidonic acid and do not contain ring structure. - They are formed in Leukocytes , Platelets and macrophage . - They are involved in inflammation, chemotaxis (attraction of leukocytes to site of inflammation) and allergic effect. - The slow-reacting substance of anaphylaxis (SRS-A) is a mixture of leukotrienesC4, D4 and E4. This mixture is 100-1000 times more potent than histamine or PG as a constrictor of the bronchial airway musculature . - Leukotrienes B4 (LTB4) attract leukocytes ( neutrophils and eusinophils ) , which are found in large number at site of inflammation . Lipoxins ( LX ) : - Lipoxins are a family of conjugated tetraenes also arising in leukocytes. They are formed by the combined action of more than one lipooxygenase. - Evidence supports a role of LX's in vasoactive and immunoregulatory function, e.g.. as counter-regulator compounds ( chalones ) of the immune response . 144