CHAPTER 16
Carboxylic Acids, Esters,
and Other Acid Derivatives
Test Bank
TYPE I MULTIPLE-CHOICE QUESTIONS
In each of the following multiple-choice questions, place the letter of the correct response in the blank
at the left. There is only one correct response for each question.
16.1
b
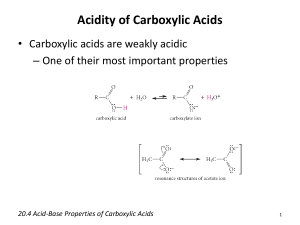
The functional group present in carboxylic acids is called a
a) carbonyl group.
b) carboxyl group.
c) carboxylate group.
d) carbohydroxyl group.
16.2
c
Which of the following statements concerning the structure of the functional group
present in carboxylic acids is incorrect?
a) Two oxygen atoms are present.
b) A carbon-oxygen single bond and a carbon-oxygen double bond are present.
c) A carbon-hydrogen bond is present.
d) An oxygen-hydrogen bond is present.
CH3
16.3
176
c
The IUPAC name for the compound CH3 CH
a) 2-methyl-4-butanoic acid.
b) 2-methyl-4-pentanoic acid.
c) 3-methylbutanoic acid.
d) 3-methylpentanoic acid.
O
CH2 C
OH is
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Copyright Houghton Mifflin Company. All rights reserved.
177
178
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
16.4
d
The common names for the C2 mono- and dicarboxylic acids are, respectively,
a) formic acid and acetic acid.
b) acetic acid and formic acid.
c) oxalic acid and acetic acid.
d) acetic acid and oxalic acid.
16.5
b
[Algorithmic]The common name for the C4 unbranched dicarboxylic acid is
a) malonic acid.
b) succinic acid.
c) glutaric acid.
d) adipic acid.
16.6
d
[Algorithmic]Which of the following polyfunctional carboxylic acids is incorrectly
characterized?
a) glyceric acid, C3 dihydroxyacid
b) lactic acid, C3 hydroxyacid
c) pyruvic acid, C3 ketoacid
d) fumaric acid, C4 ketodiacid
16.7
c
Which of the following statements concerning the properties of carboxylic acids is
incorrect?
a) Short-chain monocarboxylic acids are liquids at room temperature.
b) Carboxylic acids have higher boiling points than alcohols of similar molecular
mass.
c) Carboxylic acids cannot hydrogen bond to water.
d) Carboxylic acids can hydrogen bond to each other.
16.8
c
[Algorithmic]Carboxylic acids may be prepared by oxidation of either
a) aldehydes or ketones.
b) primary or secondary alcohols.
c) aldehydes or primary alcohols.
d) aldehydes or secondary alcohols.
16.9
a
[Algorithmic]Which of the following statements concerning the acid strength of
carboxylic acids is correct?
a) All are weak acids.
b) All are strong acids.
c) Nonaromatic acids are weak and aromatic acids are strong.
d) Unbranched acids are weak and branched acids are strong.
16.10
b
[Algorithmic]Which of the following statements concerning carboxylate ions is
correct?
a) They may be positively or negatively charged.
b) They contain fewer hydrogen atoms than their parent acids.
c) They contain more oxygen atoms than their parent acids.
d) They are always positively charged.
Test Bank
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
179
16.11
b
[Algorithmic]Carboxylic acid salts may be converted to their “parent” carboxylic
acids by reacting them with
a) a strong base.
b) a strong acid.
c) an alcohol.
d) an aldehyde.
16.12
b
Which of the following acids would produce a carboxylate ion with a minus two
charge?
O
a) CH3 C
OH
O
O
C
C
O
O
c) CH3 C
C
b) HO
OH
OH
OH O
d) CH3 CH C
16.13
c
OH
Which of the following is a general structural representation for an ester?
O
a) R
C
O H
O
b) R C
R
O
c) R
C
O R
O
d) R C
16.14
b
H
[Algorithmic]Esters are produced through the reaction of
a) an alcohol and an aldehyde.
b) an alcohol and a carboxylic acid.
c) a carboxylic acid and an aldehyde.
d) a carboxylic acid and a ketone.
Copyright Houghton Mifflin Company. All rights reserved.
180
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Test Bank
O
16.15
b
The IUPAC name for the ester CH3 CH2 CH2 C O CH3 is
a) butyl methanoate.
b) methyl butanoate.
c) methyl butyl ester.
d) methyl carboxybutane.
16.16
b
[Algorithmic]The ester obtained by reacting ethanol and benzoic acid is called
a) ethyl benzene.
b) ethyl benzoate.
c) phenyl ethanoate.
d) benzyl ethanoate.
16.17
d
When an ester is saponified, the reaction products are
a) water and a base.
b) a carboxylic acid and an alcohol.
c) a carboxylic acid and a carboxylic acid salt.
d) a carboxylic acid salt and an alcohol.
16.18
b
[Algorithmic]The monomers for the formation of a polyester are
a) a dicarboxylic acid and a monoalcohol.
b) a dicarboxylic acid and a dialcohol.
c) two diesters.
d) two monoesters.
16.19
d
Which of the following statements concerning acid anhydrides is incorrect?
a) They undergo hydrolysis to produce the parent carboxylic acid.
b) They are generally less reactive than the corresponding acid chloride.
c) They have structures in which three oxygen atoms are present.
d) They are produced by reacting two carboxylic acids with each other.
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
16.20
c
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
181
Which of the following is the general formula for a phosphate monoester?
O
a) R
O
P
OH
O
b) R
O P
O
OH
R
O
c) R O
P
OH
OH
O
d) R
O P
O
R
O R
TYPE II MULTIPLE-CHOICE QUESTIONS
In each of the following multiple-choice questions place the letter of the correct response in the blank
at the left. There may be more than one correct response for a question (choice d) or no correct
response for a question (choice e).
16.21
d
Which of the following is a correct notation for the carboxylic acid functional group?
O
a)
C
OH
b)
COOH
c)
CO2H
d) more than one correct response
e) no correct response
Copyright Houghton Mifflin Company. All rights reserved.
182
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
16.22
b
Test Bank
Which of the following carboxylic acids is paired with its correct IUPAC name?
Cl
a) CH3 CH
O
CH2 C
Cl
b) CH3 CH2 CH
c) CH3
OH ; 2-chloro-4-butanoic acid
O
C
Cl
Cl
O
CH
CH
C
OH ; 2-chlorobutanoic acid
OH ; 2,3-dichloro-1-butanoic acid
d) more than one correct response
e) no correct response
16.23
a
In which of the following pairs of carboxylic acids are both members of the pair
dicarboxylic acids?
a) malonic acid and succinic acid
b) glutaric acid and butyric acid
c) oxalic acid and acetic acid
d) more than one correct response
e) no correct response
16.24
e
For which of the following biochemically important polyfunctional carboxylic acids
is its accompanying characterization correct?
a) fumaric acid; a keto acid
b) pyruvic acid; an unsaturated acid
c) lactic acid; a dihydroxy acid
d) more than one correct response
e) no correct response
16.25
d
Which of the following functional groups is always found at the end of a carbon
chain?
a) aldehyde
b) carboxylic acid
c) ketone
d) more than one correct response
e) no correct response
16.26
a
Which of the following statements concerning the boiling points of carboxylic acids
is correct?
a) They are higher than those of corresponding alcohols.
b) They are lower than those of corresponding alkanes.
c) They are lower than those of corresponding aldehydes.
d) more than one correct response
e) no correct response
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
183
16.27
c
Which of the following compounds could not be oxidized to a carboxylic acid?
a) propanal
b) 2-methyl-1-propanol
c) 2-methyl-2-propanol
d) more than one correct response
e) no correct response
16.28
d
Which of the following carboxylic acids possesses two acidic hydrogen atoms?
a) butanedioic acid
b) 2-methylbutanoic acid
c) succinic acid
d) more than one correct response
e) no correct response
16.29
b
In which of the following pairs of compounds does the first listed compound have a
greater solubility in water than the second listed compound?
a) hexanoic acid, methanoic acid
b) sodium butanoate, butanoic acid
c) pentanoic acid, potassium pentanoate
d) more than one correct response
e) no correct response
16.30
d
The general structural difference between a carboxylic acid and an ester is the same as
that between an
a) alcohol and an ether.
b) aldehyde and a ketone.
c) alkane and an alkene.
d) more than one correct response
e) no correct response
16.31
d
Which of the following esters is paired with an incorrect IUPAC name for it?
O
a) CH3 CH2 C O CH3 ; ethyl methanoate
O
b) CH3 C O CH2 CH3 ; methyl ethanoate
O
c) CH3 CH2 C O CH2 CH3 ; ethyl propanoate
d) more than one correct response
e) no correct response
Copyright Houghton Mifflin Company. All rights reserved.
184
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
16.32
b
Which of the following pairings of ester common and IUPAC names is correct?
a) ethyl propionate and propyl ethanoate
b) propyl acetate and propyl ethanoate
c) ethyl butyrate and butyl ethanoate
d) more than one correct response
e) no correct response
16.33
c
Which of the following compounds is a constitutional isomer of ethyl ethanoate?
a) methyl methanoate
b) ethyl propanoate
c) butanoic acid
d) more than one correct response
e) no correct response
16.34
d
For which of the following types of carboxylic acid derivatives is the parent
carboxylic acid(s) a product of the derivative’s hydrolysis?
a) acid anhydride
b) acid chloride
c) ester
d) more than one correct response
e) no correct response
16.35
c
Which of the following types of inorganic esters would contain two oxygen-carbon
single bonds?
a) diphosphate monoester
b) phosphate triester
c) diphosphate diester
d) more than one correct response
e) no correct response
Test Bank
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
185
MULTIPLE-CHOICE FORMAT TRUE-FALSE QUESTIONS
In each of the following multiple-choice questions, characterize EACH of the three given statements
as being TRUE or FALSE and then indicate the collective true-false status of the statements using the
choices
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.36 b - TTF Statements:
(1) The compound ethanoic acid contains two carbon atoms and two oxygen
atoms.
(2) Unsubstituted short-chain monocarboxylic acids are completely miscible with
water.
(3) The two reactants in an esterification reaction are a carboxylic acid and an
aldehyde.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.37 c - FFT
Statements:
(1) The boiling points of esters are much higher than those of corresponding
carboxylic acids.
(2) The compound benzoic acid is a heterocylic compound.
(3) In linear form, the ester functional group can be represented as -COOR.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.38 a - TTT
Statements:
(1) The compound 4-oxobutanoic acid contains both a carboxyl group and a
carbonyl group.
(2) Aldehyde oxidation is a method for preparing a carboxylic acid.
(3) Both IUPAC and common names for esters consist of two words.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
Copyright Houghton Mifflin Company. All rights reserved.
186
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Test Bank
16.39 d - FFF Statements:
(1) A carboxylic acid and its corresponding carboxylate ion differ in the number
of oxygen atoms they contain.
(2) In ester hydrolysis, the carbon-oxygen double bond breaks producing an
alcohol and a carboxylic acid.
(3) The acronym PET stands for polyester(terephthalate).
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.40 d - FFF Statements:
(1) Hydrogen bonding is not possible between carboxylic acid molecules.
(2) The alpha-carbon atom in a carboxylic acid is the carbon atom to which the
carboxyl group is attached.
(3) Sodium butanoate and ethyl butanoate are both examples of esters.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.41 b - TTF Statements:
(1) Structurally, the difference between malonic acid and succinic acid is a -CH2grouping.
(2) A carboxylic acid salt is generally more soluble in water than the carboxylic
acid from which it is obtained.
(3) Ester hydrolysis and ester saponification are “reverse” reactions of each other.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.42 b - TFT
Statements:
(1) “Pleasant odor” is a general characteristic of esters.
(2) Citric acid is both a tricarboxylic acid and a ketoacid.
(3) Esters and carboxylic acids with the same number of carbon atoms and the
same degree of saturation are constitutional isomers.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
16.43 b - TFT
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
187
Statements:
(1) Hydrolysis of a mixed acid anhydride produces two different carboxylic acids
as products.
(2) Short-chain unsubstituted monocarboxylic acids are strong acids while their
longer-chain counterparts are weak acids.
(3) The parent alcohol for the ester methyl acetate is methyl alcohol.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.44 a - TTT
Statements:
(1) Pyruvic acid is the keto-derivative of propanoic acid.
(2) Reaction of a carboxylic acid with a thiol produces a thioester, a compound
containing two carbon-sulfur single bonds.
(3) One of the products of an esterification reaction is water.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
16.45 c - FTF
Statements:
(1) Nitroglycerin is an example of a phosphate triester.
(2) In the body, aspirin is converted to salicylic acid, which is the “active”
ingredient.
(3) Both ibuprofen and aspirin are propanoic acid derivatives.
a) All three statements are true.
b) Two of the three statements are true.
c) Only one of the statements is true.
d) None of the statements is true.
Copyright Houghton Mifflin Company. All rights reserved.
188
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Test Bank
MATCHING QUESTIONS
For each pair of compounds on the left, select a correct characterization from the response list on the
right. Responses on the right may be used more than once or need not be used at all.
16.46
a
Methyl propanoate
Propyl methanoate
16.47
d
Acetic acid
Ethanoic acid
16.48
c
Butanedioic acid
Butanoic acid
16.49
c
Benzoic acid
Propyl butanoate
16.50
a
2-Methylpentanoic acid
-Methyl valeric acid
a) have the same molecular formula but are
different compounds
b) both contain a carbon ring system
c) contain the same number of carbon atoms
but are not constitutional isomers
d) two names for the same compound
For each of the compound characterizations on the left, select from the response list on the right the
number of constitutional isomers that exist. Responses on the right may be used more than once or
need not be used at all.
16.51
c
Saturated four-carbon ester
16.52
a
Saturated four-carbon monocarboxylic acid
16.53
a
Saturated four-carbon dicarboxylic acid
16.54
b
Chlorobenzoic acid
16.55
b
Molecular formula of C3H6O2
a)
b)
c)
d)
two
three
four
five
Copyright Houghton Mifflin Company. All rights reserved.
Test Bank
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
189
For each pair of carboxylic acids on the left, select a correct characterization from the response list on
the right. Responses on the right may be used more than once or need not be used at all.
16.56
a
Fumaric acid
Succinic acid
16.57
c
Citric acid
Lactic acid
16.58
b
Glyceric acid
Pyruvic acid
16.59
d
Valeric acid
Caproic acid
16.60
a
Oxalic acid
Glutaric acid
a)
b)
c)
d)
Both are dicarboxylic acids.
Both are C3 acids.
Both are monohydroxy acids.
Both are unsubstituted monocarboxylic acids.
Identify the missing reactant in each of the reactions on the left using the response list on the right.
Responses on the right may be used more than once or need not be used at all.
16.61
a
Acid + ?
carboxylate ion + H3O+
16.62
b
Acid + ?
acid salt + H2O
16.63
c
Acid + ?
ester + H2O
16.64
b
Ester + ?
acid salt + alcohol
16.65
a
Ester + ?
acid + alcohol
Copyright Houghton Mifflin Company. All rights reserved.
a)
b)
c)
d)
water
base in water
alcohol
carboxylic acid
190
Chapter 16: Carboxylic Acids, Esters, and Other Acid Derivatives
Test Bank
For each of the inorganic esters on the left, select from the response list on the right the combination
of reactants needed to produce the ester. Responses on the right may be used more than once or need
not be used at all.
O
16.66
d
RO P
O
O
OH
P
OH
O
16.67
b
RO P
O
O
a)
P OH
b)
OH
c)
d)
OR
one acid molecule
one alcohol molecule
one acid molecule
two alcohol molecules
two acid molecules
one alcohol molecule
three acid molecules
one alcohol molecule
OH
O
16.68
a
HO S OR
O
O
16.69
a
RO N
O
O
CH2 O N
O
O
16.70
d__
CH O N
O
O
CH2 O N
O
Copyright Houghton Mifflin Company. All rights reserved.