Solid - Liquid - Vapor equilibria: phase diagrams
advertisement
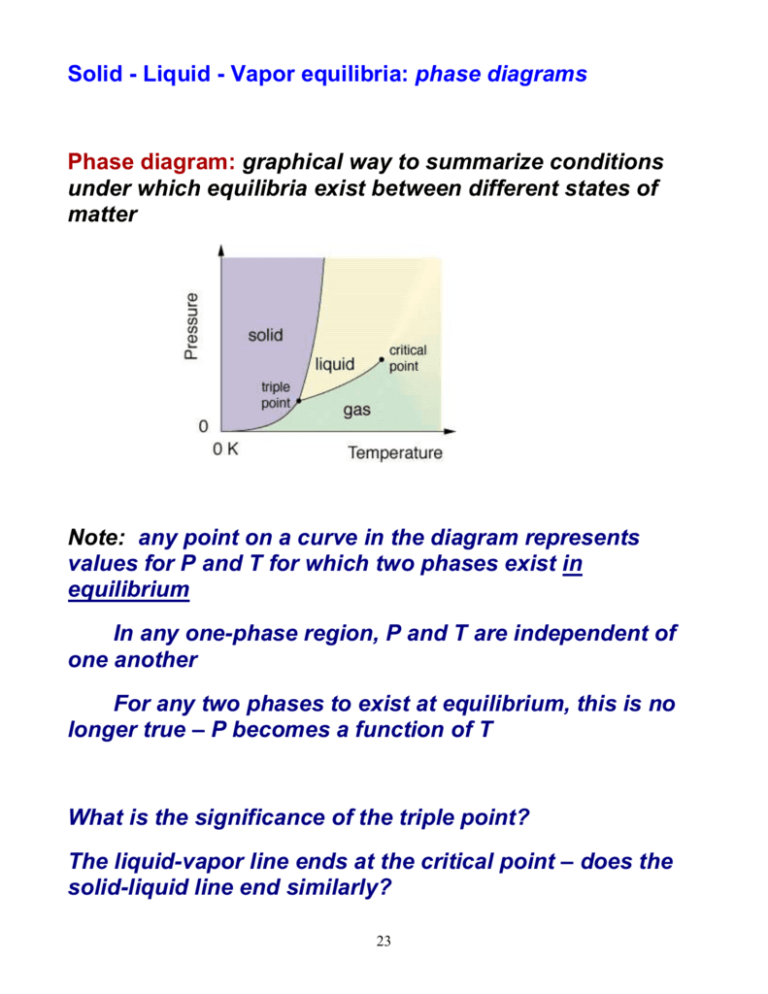
Solid - Liquid - Vapor equilibria: phase diagrams Phase diagram: graphical way to summarize conditions under which equilibria exist between different states of matter Note: any point on a curve in the diagram represents values for P and T for which two phases exist in equilibrium In any one-phase region, P and T are independent of one another For any two phases to exist at equilibrium, this is no longer true – P becomes a function of T What is the significance of the triple point? The liquid-vapor line ends at the critical point – does the solid-liquid line end similarly? 23 Phase diagrams for H2O and CO2: Fig 11.27 (text) Note that the solid-liquid line in the H2O phase diagram slopes to the left; why? At 1 atm pressure and room temperature, would CO2 melt or sublime? Why? E.g., The normal melting and boiling points of Xe are -112oC and -107oC. The triple point of Xe is -121oC and 282 torr and its critical temperature and pressure are 16.6oC and 57.6 atm. Sketch the phase diagram for Xe. Will Xe(s) float on Xe(l)? If Xe gas is cooled under an external pressure of 100 torr, will it undergo condensation or deposition? 24 Structures of Solids How do properties of solids relate to their structures and bonding? Types of solids Crystalline solids Atoms, ions, or molecules ordered in a well-defined arrangement Have highly regular shapes Intermolecular forces uniform throughout – melt at a specific temperature E.g., crystalline SiO2 25 Amorphous solids (noncrystalline) Particles have no regular, orderly structure Intermolecular forces vary in strength throughout sample – soften/melt over a range of temperatures Examples: rubber, SiO2 glass (shown here) 26 Notice: in the crystalline solids, there is a small unit of atoms that ‘repeats’ throughout the structure The unit cell is the smallest repeating unit which shows the symmetry of the entire solid What is the smallest repeating unit in the crystalline SiO2 example above? The unit cell can be used to generate the threedimensional structure of the crystal, or the crystal lattice Three types of cubic unit cells (we restrict ourselves to the cubic type): Primitive cubic: lattice points (atoms/ions) at corners only, e.g., 27 Body-centered cubic (Bcc): lattice point at corners & center of cell, e.g., Face-centered cubic (Fcc): lattice points at corners & centers of each face, e.g., 28 What is the net number of particles in each type of unit cell? Each unit cell is a part of the total three-dimensional lattice and is thus shared..... primitive cubic unit cell 8 corners x 1/8 particle/corner = 1 particle body-centered cubic unit cell 8 corners x 1/8 particle/corner + 1 center x 1 particle/center = 2 particles face-centered cubic unit cell 8 corners x 1/8 particle/corner + 6 faces x 1/2 particle/face = 4 particles Recall: density is an intensive property of matter – it is a ratio of extensive properties Since density doesn’t depend on amount, the density of a unit cell is the same as the density of a large sample of substance! 29 E.g., Cu crystallizes in an FCC unit cell whose edge length is 3.61 Å. Calculate the density of Cu metal. E.g., Iridium crystallizes in an FCC unit cell that has an edge length of 3.833 Å. The atom in the center of each face is in contact with the corner atom. Calculate the atomic radius of an Ir atom. Calculate the density of Ir metal. 30 Notice: most metals have simple cubic unit cells with only one atom at each lattice point E.g. Ni has the Fcc structure; Na has the Bcc unit cell. Structures are pretty easy to think about if we treat the metal atoms as being: (a) spherical (b) all the same size Notice that the structures adopted by crystalline solids are those that bring particles in closest contact to maximize attractive forces...... Which arrangement of particles brings each particle into contact with the largest number of other particles? 31 The structure on the right is called the closest-packed structure Each particle is in contact with 6 other particles in the same layer Now: how do these layers of spheres stack? Pretty easy to stack the second layer of spheres Fit in the depressions on top of the first layer What about the third layer? Two ways to orient: In line with first layer: hexagonal close packing (hcp) Not in line with first layer: cubic close packing (ccp) Important feature: in either hcp or ccp structures, each sphere has 12 equidistant neighbors Each sphere has a coordination number of 12 Now: what about an ionic compound, where we’re packing ‘spheres’ that aren’t the same size (anions and cations)? 32 Bonding in solids Physical properties of solids (e.g. melting point, hardness, conductivity) depend both on the arrangement of particles and the attractive forces between them Molecular solids Consist of atoms or molecules held together by intermolecular forces (dipole-dipole, etc.) Usually soft, low-melting ( < 200oC); poor conductors Most substances that are gases or liquids at room temp form molecular solids, e.g., Ar, H2O Covalent network solids Atoms held in networks or chains by covalent bonds Much harder & higher melting than molecular solids e.g., SiO2, graphite, diamond Ionic solids 33 Held together by ionic bonds Strength of bonds depends upon ionic charges and radii (remember Coulomb’s law!) High-melting; poor conductors Metallic solids Consist entirely of metal atoms Bond strength intermediate between dispersion forces and covalent bonds e- delocalized throughout entire solid – excellent conductors (thermal and electrical) Wide range of hardness and melting point 34 Problems du Jour An element crystallizes in a Bcc lattice. The edge of the unit cell is 2.86Å and the density of the crystal is 7.92 g/cm3. Calculate the atomic weight of the element. Indicate the type of crystal (molecular/metallic/covalentnetwork/ionic) that each of the following would form: CaCO3 Pt ZrO2 (m.p. 2677oC) Kr C6H6 I2 35