Hw ISSUES - AHSbogna
advertisement
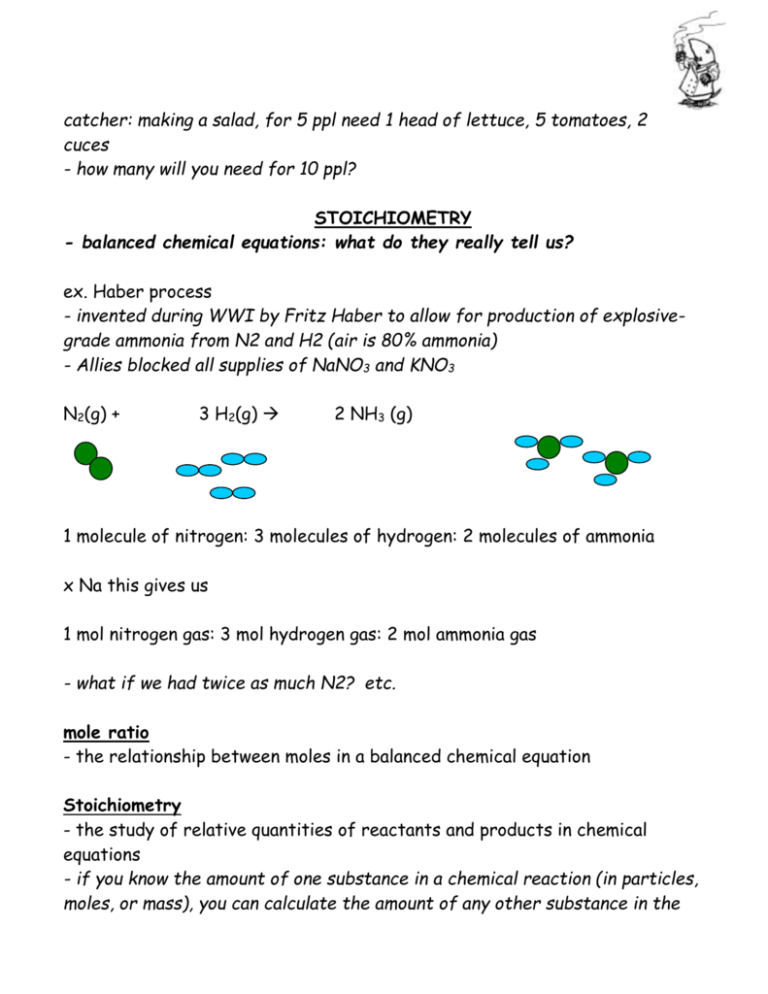
catcher: making a salad, for 5 ppl need 1 head of lettuce, 5 tomatoes, 2 cuces - how many will you need for 10 ppl? STOICHIOMETRY - balanced chemical equations: what do they really tell us? ex. Haber process - invented during WWI by Fritz Haber to allow for production of explosivegrade ammonia from N2 and H2 (air is 80% ammonia) - Allies blocked all supplies of NaNO3 and KNO3 N2(g) + 3 H2(g) 2 NH3 (g) 1 molecule of nitrogen: 3 molecules of hydrogen: 2 molecules of ammonia x Na this gives us 1 mol nitrogen gas: 3 mol hydrogen gas: 2 mol ammonia gas - what if we had twice as much N2? etc. mole ratio - the relationship between moles in a balanced chemical equation Stoichiometry - the study of relative quantities of reactants and products in chemical equations - if you know the amount of one substance in a chemical reaction (in particles, moles, or mass), you can calculate the amount of any other substance in the reaction, (in particles, moles, or mass), using the information in the balanced chemical equations Why is this important? - look at ratio of reactants, C: 02 C (s) + O2 (g) CO2 (g) 2 C(s) + O2(g) 2 CO (g) 1:1 2:1 - importance of well-ventilated spaces around furnace, etc. ex. How many moles of ammonia are produces by 2.8 mol of hydrogen gas? set up as ratio 2 mol NH3: 3 mol H2 = n mole NH3: 2.8 mol H2 ex. out in space Carbon dioxide that is produced by astronauts can be removed with lithium hydroxide. The reaction produces lithium carbonate and water. An astronaut produces an average of 1.00 x 103 g carbon dioxide per day. How much lithium hydroxide should engineers put on the spaceship per astronaut, per day? - write balanced chem. equation - convert mass to moles - use ratio to get desired reactants/products in moles - convert moles to desired units ex. A fuel mixture of hydrazine, N2H4, and dinitrogen tetroxide, N2O4 was used to launch a lunar module. These two compounds react to form nitrogen gas and water vapour. If 150.0g of hydrazine reacts with sufficient dinitrogen tetroxide, what mass of nitrogen gas is formed? Given: N2H4 (l) + N2O4 (l) N2 (g) + H2O(g) Mass of reactant, N2H4 = 150.0 g ex. sodium bicarb with HCl, how many moles of carbon dioxide gas are produced? NaHCO3 (s) + HCl (aq) NaCl (aq) + H2O(l) + CO2 (g) (strong, try 8 M) mass of reactants: mass of products: PORTFOLIO STOICHIOMETRY PRACTICE 1. In the synthesis reaction of iron and sulphur to form iron II sulphide what mass of iron is needed to react completely with 32.0 g of sulphur? (Ans. 55.7 g) 2. How much ammonium hydroxide is needed to react completely with 75.0 g of copper II nitrate in a double displacement reaction? (Ans. 28.0 g) 3. A Sundocal tablet contains 0.080 g of calcium carbonate CaCO3. When a tablet is ingested it reacts with hydrochloric acid, HCl in the stomach. What mass of HCl is needed to dissolve the CaCO3 in a tablet. (Ans. 58 mg) CaCO3 + 2HCl CaCl2 + H2O + CO2 4. A Nature's Own Calcium tablet contains 863 mg of calcium carbonate CaCO3. When a tablet is ingested it reacts with hydrochloric acid, HCl in the stomach. What mass of HCl is needed to dissolve the CaCO3 in a tablet. (Ans. 629 mg) CaCO3 + 2HCl CaCl2 + H2O + CO2











