World Journal Of Engineering STUDY OF THE Sn/C NANOFIBERS
advertisement

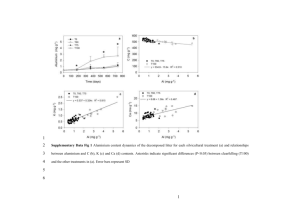
World Journal Of Engineering STUDY OF THE Sn/C NANOFIBERS WITH DIFFERENT Sn CONTENTS FOR ANODE MATERIALS IN LITHIUM-ION BATTERIES Keyu Li, Yunhua Yu, Yu Teng, Xin Li and Xiaoping Yang Key Laboratory of carbon fiber and functional polymers, Ministry of Education, Beijing University of Chemical Technology, Beijing 100029, China E-mail: yangxp@mail.buct.edu.cn Electrochemical performance measurements were performed using 2025 coin cells, which is assembled according to our previous work [4]. The electrolyte was 1 M LiPF6 in a 1:1 mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC). Charge and discharge were executed with a cells testing instrument (LAND CT2001A) at a current density of 50 mA g-1, with the cutoff potentials of 0.001 and 3V vs. Li/Li +. Introduction There is an increasing demand for new anode materials of rechargeable lithium ion batteries (LIBs) possessing high capacity and long cycle life. Sn-based materials have been focused on in recent years because their capacities (Sn 994 mAh g-1) exceed that of commercial graphite (372 mAh g-1) [1, 2]. However, there are large changes of volume associated with the formation of different Li-Sn phases. With this change, the anode materials were pulverized and the cycle performance decreased. Jung Won Park [3] fabricated the Sn-C composite electrodes with well ‘buffering effects’. Following our previous study [4], it was found that a kind of nano-sized Sn/C composite nanofibers could supply good electrochemical performance during their continued charge/discharge process. When preparing the Sn/C anode material, the content of Sn have been considered as key factor influencing their electrochemical performance. In this work, the Sn/C composite with different Sn loading were prepared by electrospinning and carbonization. The purpose of the work was to investigate the effect of Sn loading on the morphology and electrochemical performance of the as-prepared Sn/C composite anode materials. Results and Discussion Fig.1 shows SEM images of pure CNFs and the Sn/C nanofibers with different contents of Sn precursor (0.4, 0.5 and 0.6ml) in the electrospinning solutions. All nanofibers are continuous and formed a reticular morphology. The Sn/C nanofibers (Fig.1 A, B, C) were thicker than pure CNFs (Fig.1D). As increasing content of Sn, nanofibers diameter increased gradually, from 100-200nm (D) to 400-500nm (C), because of the compound intermingle. Experimental Materials PAN copolymer fibril(Mw=100, 000 g/mol, 93.0 wt.% acrylonitrile, 5.3 wt.% methylacrylate, and 1.7 wt.% itaconic acid, UK Courtaulds Co.) Tin tetrachloride (SnCl4, 98%, Alfa Aesar) ethylene glycol ((HOCH 2)2, 99%, Alfa Aesar) The amount of adding solution are 0.4, 0.5 and 0.6ml, respectively. Sn/C nanofibers were prepared through the electrospun process under an advisable temperature and humidity follow by carbonization as previous work [4]. Pure CNFs were also prepared by the same process. Fig.1 SEM images and diameter distribution insets of Sn/C nanofibers with different Sn precursor contents (A) 0.4ml, (B) 0.5 ml, (C) 0.6 ml and (D) pure CNFs carbonized at 850℃. Apparatus and Procedures The surface morphology of Sn/C nanofibers and CNFs was observed using a scanning electron microscope (SEM, zeiss supera 55, Germany). Fig.2 shows the first galvanostatic discharge/charge profiles of the Sn/C nanofibers and pure CNFs at a current density of 50 mA g-1, with the cutoff potentials of 661 World Journal Of Engineering 0.001 and 3V. With formation of solid electrolyte interface (SEI) film and the decomposition of the electrolyte, the discharge curve of the first cycle had a rapid voltage drop to 1V. The second part below 1V is related with the complicated insertion of Li + into CNFs and Sn. From the curves, the capacity of discharge process was increased with the increase of Sn. The Sn/C nanofibers content 0.6ml Sn precursor delivered higher discharge capacity (1102.6 mAh g-1) than others because of more Li+ insert. But the charge capacity of this sample decreased to 632.9 mAh g-1, corresponding to coulombic efficiency of 57.4%. The Sn/C nanofibers content 0.5ml Sn precursor exhibited higher charge capacity (671.4 mAh g-1) than others with coulombic efficiencies of 71.7%. The thicker fibers (Fig.1C) were probably too large, and might hinder the diffusion of Li+. Inserted Li+ could not be taken off completely from nanofibers due to the decrease of charge capacity. So we thought the diameter of nanofibers and the content of Sn had a trade-off effect. Fig.3 Reversible capacities versus cycles of Sn/C nanofibers with different Sn precursor content and pure CNFs carbonized at 850℃. Conclusion The Sn/C nanofibers with different Sn loading were prepared using electrospinning technique and carbonization treatment. By increasing the Sn content, the diameter of the as-prepared Sn/C nanofibers rised, while the reversible capacity exhibited a point of inflection. The Sn/C nanofibers with 0.5mL Sn precursor solution showed the highest reversible capacity and excellent cycling performance, with a capacity rentation of 520 mAh g-1 after 30 cycles at 50 mA g-1. References 1.Besenhard, J.O., Yang, J. and Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries. J. Power Sources, 68 (1997) 87-90. Fig.2 Galvanostatic discharge/charge curves for the first cycle of Sn/C nanofibers with different Sn content an pure CNFs carbonized at 850℃. 2.Zou, L., Gan, L., Kang, F. Y., Wang, M. X., Shen, W. C. and Huang, Z. H. Sn/C non-woven film prepared by electrospinning as anode materials for lithium ion Fig.3 shows reversible capacities of Sn/C nanofibers with different Sn content and pure CNFs carbonized at 850℃. Although the discharge capacities decreased in the first cycles, all the Sn/C nanofibers anodes had much higher reversible capacities than that of pure CNFs due to the loading of Sn. At the 30th cycle, their reversible capacities decreased to about 480, 520, 450mAh g-1, showing that the Sn/C nanofibers with 0.5ml Sn precursor content presented the highest capacity. batteries. J. Power Sources, 195 (2010) 1216–1220. 3.Park, J. W., Eom, J. Y. and Kwon, H. S. Fabrication of Sn–C composite electrodes by electrodeposition and their cycle performance for Li-ion batteries, Electrochemistry Commun., 11 (2009) 596–598. 4.Yu, Y. H., Yang, Q., Teng, D. H., Yang, X. P. and Ryu, S. K. Reticular Sn nanoparticle-dispersed PAN-based carbon nanofibers for anode material in rechargeable lithium-ion batteries, Electrochemistry Commun., 12 (2010) 1187–1190. 662