Fabrication and Photocatalytic Activities of SrTiO 3 Nanofibers by
advertisement
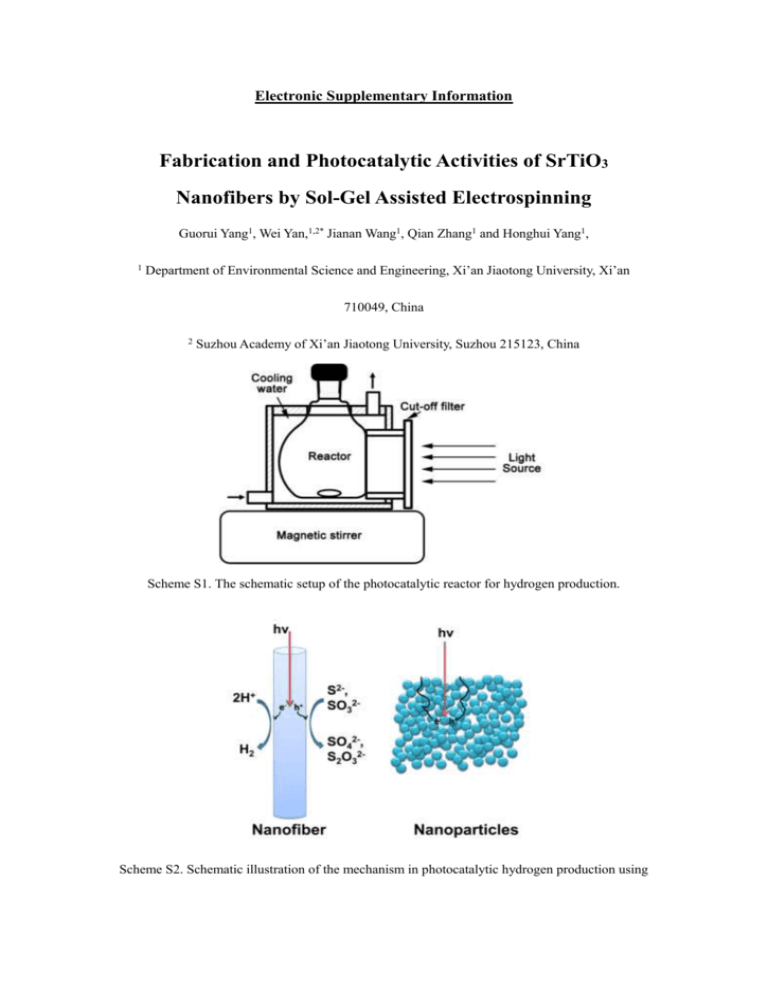
Electronic Supplementary Information Fabrication and Photocatalytic Activities of SrTiO3 Nanofibers by Sol-Gel Assisted Electrospinning Guorui Yang1, Wei Yan,1,2* Jianan Wang1, Qian Zhang1 and Honghui Yang1, 1 Department of Environmental Science and Engineering, Xi’an Jiaotong University, Xi’an 710049, China 2 Suzhou Academy of Xi’an Jiaotong University, Suzhou 215123, China Scheme S1. The schematic setup of the photocatalytic reactor for hydrogen production. Scheme S2. Schematic illustration of the mechanism in photocatalytic hydrogen production using the nanofibers and nanoparticles. The advantages of nanofibers over nanoparticles during the photocatalytic process have been shown in Scheme S2. As is well known, Photocatalysts must be thick enough to absorb incident light efficiently. Nanofibers have a long axis to absorb incident light, and orthogonally, a short radial axis to separate charge. However, the photogenerated charge carriers in the nanoparticle photocatalysts must undergo a relatively longer migration distance, which retard the photocatalytic reducetion and oxidation reaction. Moreover, the boundaries between different nanoparticles also act as recombination centers for photogenerated charge carriers, resulting the reduction of photocatalytic efficiency. Therefore, the high boundary density in nanoparticle photocatalyst has a negative influence on the photocatalytic water splitting and degradation over RhB. During the photocatalytic process, hydrogen is produced (Equation 1 and Scheme S2), and the sulfide and sulfite anions act as sacrificial reagents which is consumed by photogenerated holes (Equations 2-5 and Scheme S2). Different reactions occur for the photogenerated electrons and holes as follows: 2H++2eSO32- + 2OH- + 2h+ 2S2- + 2h+ S22- + SO32SO32- + S2- + 2h+ H2 1 SO42- + 2H+ 2 S22S2O32-+ S2S2O32- 3 4 5 As illustrated in equations 2 and 3, photoinduced holes react with SO32- and S2- to yield SO42- and S22-, respectively. The production of S22- ions may play as a depressing electron acceptor capturing the photoexcited electrons, so hinder the water from being reduced. This process can be suppressed in the presence of SO32- ions by equation 4, and the anions of S2- and S2O32- are introduced. The ions of S2O32- is colorless that can avoid the decrease of the light absorption efficiency. More importantly, S2O32- is inactive in the reduction process, which has a little negative effect on the reaction. Equation 5 represented the main working process of the sacrificial reagents. Fig. S1 SEM images of P25 with different magnifications. Fig. S2 TG analysis of SrTiO3 precursor fibers In order to display the decomposition process of the precursor fibers, TG analysis was carried out. As illustrated in Fig. S2, the TG curve elucidates three obvious weight loss processes. The first mass loss is about 11.9 % before 210 °C, which is resulted from evaporation of acetic acid, methyl alcohol and a little water. The second obvious weight loss (ca.54.5 %) between 210 and 445 °C is mainly ascribed to the decomposition of zinc acetate and manganese acetate and PVP. When the temperature is higher than 450 °C, no discernible weight loss was observed indicating that the precursor is converted to SrTiO3 nanofibers completely.