UNIT: ACIDS, BASES AND METALS
advertisement

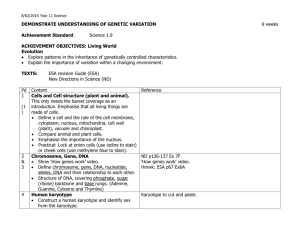
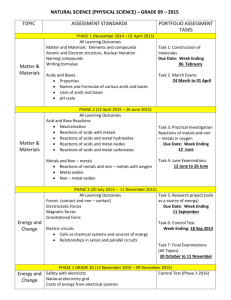
UNIT: DEMONSTRATE AN UNDERSTANDING OF CHEMICAL IDEAS RELATING TO ACIDS AND BASES ACHIEVEMENT STANDARD: Science 1.5 ACHIEVEMENT OBJECTIVES: Material World Properties and changes of matter Identify patterns and trends in the properties of a range of groups of substances, for example, acids and bases, metals, metal compounds and hydrocarbons. Explore factors that affect chemical processes. The structure of matter Distinguish between atoms, molecules, and ions (includes covalent and ionic bonding). Link atomic structure to the organisation of the periodic table. Use particles theory to explain factors that affect chemical processes. Chemistry and society Investigate how chemical knowledge is used in a technological application of chemistry. TEXTS: New Directions in Science (ND) NCEA 1 Science Hannay, Howison, Sayes (ESA) N = notes, Ex = exercises, P = practical, T = task sheet, O = OHT Pd Content 1 Periodic Table Periodic tables (1) Activity: Shade metals, non-metals, solids, liquids, gases, halogens, inert gases, Group 1, Group 2 Show video “The Periodic Table" 2 (1) 3 Structure of atoms Describe arrangement and nature of protons, neutrons, and electrons. Calculate number of protons, neutrons and electrons from atomic number and mass number. Define and explain isotopes. Electron arrangement Discuss stability of electron arrangement. Resources ND p34-37 Ex 1A, 1B Prac1 p1, Prac5 p2 Photocopy of periodic table. “The Periodic Table” video. Hmwk: ESA p196-198 ND p42 Ex 1E Hmwk: ESA p193-196 ND p44 Ex 1F (1) 4 (1) 5-6 (2) 7 Write electron arrangements for atoms and ions of the first 20 elements. Define what an ion is. Cover ion formation in the next lesson. Relate charge of monatomic (1 atom) ions to position in periodic table (Groups 1, 2, 16, 17 only). How ions form Use Ionic bond/covalent bonding Powerpoint to explain the following concepts: 1. Transfer of electrons. 2. How the charge on the ion is related to how many electrons are gained or lost. 3. Ionic bond formation. Writing formulae of ionic compounds Balancing charges when forming salts. Write formulae for AB, A2B, AB2, AB3 and salts that require brackets e.g. Ca(OH)2. Making acids and bases Introduce litmus and universal indicator as acid-base indicators. Practical: Burn Mg and dissolve ash in small amount of water. Test with ND p46 Ex 1G K:\2010 Science\Yr 11 Science\Chemistry\Powerpoints\Ion formation and ionic bonds Model of sodium chloride crystal lattice in storeroom with molymods. Hmwk: p199-201 ND p48-51 Ex 1H-1I Prac10 p3 Ion table to stick in notes. Hmwk: ESA p201-208 ND p53 Ex2A ND p55 Ex2B ND p61 Ex2E ND p65 Ex2G Prac21 p6 a few drops of Universal Indicator solution. Demonstration: Burn sulphur in gas jar of oxygen/or air. Gas jar should have a few cm of water with a few drops of Universal indicator. Test gas with damp litmus. Shake up water to dissolve gas. Observe colour Hmwk: ESA p215-217 change. OHTs (metal oxides, non-metal oxides). Save water from sulfur exp. for later lesson. 8 Explain what the colour change for each indicator means. Write equations for each of the reactions. Mg burning to produce MgO, MgO reacting with water to make Mg(OH)2, Mg(OH)2 dissolving in water (albeit only slightly) to give OHions. S burning to make sulfur dioxide, sulfur. Acid rain — oxides of sulfur and nitrogen. T Acid Rain Recognise acid nature of H+ ions and basic nature of OH- ions Conclusion: Metal oxides are basic & Non-metal oxides are acidic. pH U.I solution 9 10 Compare pH of different solutions. Write equations for dissociation of different acids Soil pH For achieved: word equations For merit: word and symbol equations For excellence: write a balanced symbol equation What can react with an acid? Reactions (1) Metal and acid (acids are limited to HCl and H2SO4) React metals (not calcium or lithium) with dilute hydrochloric acid 12 ND p74 Ex3C Prac32 p9 Hmwk: ESA p228 Write word equations [METAL + ACID —> SALT + HYDROGEN] Reactions (2) Acid and base Metal hydroxides / oxides + acids [ACID + BASE —> SALT + WATER] Practical: Add 2M acid to 2M base and record temp. change(prove ND p74 Ex 3C ND p66 Ex 2H Prac12 p4 Hmwk: ESA p235 reaction has taken place). Practical: Add U.I to ethanoic acid and add NaOH drop by drop. U.I changes to green then purple. Proves neutralisation. Practical: Add sulphuric acid to copper(II) oxide and leave over night. Copper sulphate solution produced. Proves oxides act as bases. Boil off some water and allow the crystallize. 11 Variety of household and lab solutions ND p54-55 Prac 14 & 15 p4 Hmwk: ESA p213-218 Reactions (3) Acid and carbonate/hydrogen carbonate ND p56 Ex 2C Metal carbonate / hydrogen bicarbonate + acid: observe reaction Prac18 P5 of calcium carbonate (marble chip) and hydrochloric acid. Identify gas produced (limewater). [ACID + CARBONATE —> SALT + WATER + CARBON DIOXIDE] Antacid investigation ND p56 Ex2H Carry out investigation into which antacid tablet neutralizes the most acid. This involves a simple titration or add acid bit by bit to tablet and then measure total volume used. Follow the method on sheet/OHT in folder. Students fill in results etc in 13 14 15 16 16 17 18 19 workbook 140-141. Reaction rate Effect of increasing concentration. Compare time taken to fill a boiling tube with gas at different concentrations. Discuss concentration as number of particles in a given volume. Increase conc. = more particles so more collisions. Effect of increasing temperature. Carry out thiosulfate/HCl clock reaction at different temperatures or do ND Prac34. Discuss temperature as being the average Ek of particles, so higher temp. = faster moving particles so more collisions. Effect of increasing surface area. Compare time taken for Mg to disappear when reacted with HCl (single strip vs cut up pieces). Or do ND Prac19. Discuss increased surface area = more chance of a collision. Graphing a reaction curve Carry out reaction of Mg with acid measuring volume of gas produced over time. Graph the results. Explain shape of graph. Collision theory Particles need to collide with enough energy (to overcome activation energy barrier) and with correct orientation for a successful collision to occur. Real life applications Discuss, complete problems or research any of the following: Acid rain effect on buildings Treating stings from various organisms. Using kindling to start fires. Revision Practise questions from old exam papers Test ND Prac33 p10 ND Prac34 p10 ND Prac19 p6 ND p76 3D Hmwk: p241-246 On K-drive or from NZQA website