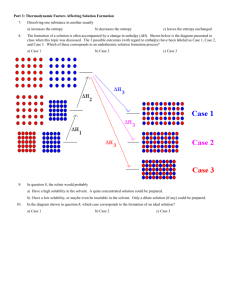
5th Grade Dissolution Rates - University of Central Oklahoma
advertisement

Dissolving Rate Inquiry Lesson For more great info, go to http://www.alka-seltzer.com/as/experiment/student_experiment.htm. There are a variety of student experiments lined out there. I wanted to do the Alka-Rockets Background There are many different factors that affect the dissolving rate of a substance. Some of the factors that make a difference in the dissolving rate of a substance are the concentration of the liquid, any movement that is added, the temperature, surface area of the solute, the kind of matter being dissolved, and the mass or volume of the substance. Students will learn about each of these factors through investigations and inquiry. The teacher must have extensive background knowledge of the factors of dissolving so that they can assist students in their experiments and answer questions that may be addressed. The teacher should already understand the following facts, and the students will learn these through their experiments. This lesson follows the following Oklahoma PASS content standard and objective: PHYSICAL SCIENCE Grade 5 Standard 1: Properties of Matter and Energy - Describe characteristics of objects based on physical qualities such as size, shape, color, mass, temperature, and texture. Energy can produce changes in properties of objects such as changes in temperature. The student will engage in investigations that integrate the process standards and lead to the discovery of the following objectives: 1. Matter has physical properties that can be used for identification (e.g., color, texture, shape). First, concentration of the solvent will affect the rate at which the solute dissolves. As the concentration of the liquid doubles, the dissolving rate doubles as well. This means that the more concentrated the solvent, the faster the solute will dissolve. 1 Dissolving Rate Inquiry Lesson Second, any movement added to the experiment will assist the dissolving rate of the substance. Both stirring and shaking accelerate the dissolving process. When movement is added, the surface area of the substance increases as it is broken down into smaller pieces. Also, there is more contact between the solute and the solvent. Movement creates kinetic energy which raises the temperature as well as the dissolving rate. Third, temperature can affect the dissolving rate of matter. An increase in temperature will increase the dissolving rate of the substance. It causes the molecules to move more rapidly which increases the kinetic energy. This allows the solute to break down even more, and the molecules come into contact with more of the solute molecules. When the temperature is lowered, the molecules move more slowly and create less energy. This means that the molecules come into contact with fewer solute molecules and do not dissolve as quickly. Fourth, the size of the surface area will influence the dissolving rate. The larger the surface area, the more solvent can touch the substance and increase dissolving. If the surface area is smaller, not as much solvent can touch the substance, which decreases the dissolving rate. Fifth, the matter being dissolved will affect the dissolving rate. Some matter is impossible to dissolve and therefore is insoluble. Some substances naturally dissolve more quickly than others. An example of this is that sugar will dissolve more quickly than a stone. Finally, the mass or volume of the substance has an effect on the dissolving rate. The larger the mass of an object, the more time it will take to dissolve. Or if there is less volume of liquid to place the object in, the dissolving rate will also slow down. While the students perform the various experiments in the lesson, they will practice a few process skills from the Oklahoma PASS objectives such as observe and measure, classify, experiment, interpret and communicate, and use inquiry tools. 2 Dissolving Rate Inquiry Lesson SCIENCE PROCESSES AND INQUIRY Grade 5 Process Standard 1: Observe and Measure - Observing is the first action taken by the learner to acquire new information about an object, organism, or event. Opportunities for observation are developed through the use of a variety of scientific tools. Measurement allows observations to be quantified. The student will accomplish these objectives to meet this process standard. 2. Compare and/or contrast similar and/or different characteristics (e.g., color, shape, size, texture, sound, position, change) in a given set of objects, organisms, or events. Process Standard 2: Classify - Classifying establishes order. Objects, organisms, and events are classified based on similarities, differences, and interrelationships. The student will accomplish these objectives to meet this process standard. 2. Arrange objects, organisms, and/or events in serial order (e.g., least to greatest, fastest to slowest). Process Standard 3: Experiment - Experimenting is a method of discovering information. It requires making observations and measurements to test ideas. The student will accomplish these objectives to meet this process standard. 1. Ask questions about the world and formulate an orderly plan to investigate a question. 2. Evaluate the design of a scientific investigation. Process Standard 4: Interpret and Communicate - Interpreting is the process of recognizing patterns in collected data by making inferences, predictions, or conclusions. Communicating is the process of describing, recording, and reporting experimental procedures and results to others. Communication may be oral, written, or mathematical and includes organizing ideas, using 3 Dissolving Rate Inquiry Lesson appropriate vocabulary, graphs, other visual representations, and mathematical equations. The student will accomplish these objectives to meet this process standard. 1. Report data using tables, line, bar, trend, and/or simple circle graphs. 3. Make predictions based on patterns in experimental data. 4. Communicate the results of investigations and/or give explanations based on data. Process Standard 5: Inquiry - Inquiry can be defined as the skills necessary to carry out the process of scientific or systemic thinking. In order for inquiry to occur, students must have the opportunity to ask a question, formulate a procedure, and observe phenomena. The student will accomplish these objectives to meet this process standard. 1. Use different ways to investigate questions and evaluate the fairness of the test. 2. Use a variety of measurement tools and technology. 3. Formulate a general statement to represent the data. 4. Share results of an investigation in sufficient detail so that data may be combined with data from other students and analyzed further. Students will “observe and measure” by looking at a variety of characteristics of dissolving alka-seltzer tablets and decide which ones affect the substance more than others. They will “classify” by taking the traits that they have observed, and put them in different orders, such as most powerful to least powerful. They will “experiment” by asking questions to predict which of the concepts has the most affect on the dissolving rate, followed by evaluating their thoughts when they are finished with the experiments. They will “interpret and communicate” by making a final graph of their findings to share with the class and be able to predict how the factors that they test will affect other substances. Students will use “inquiry” by learning through 4 Dissolving Rate Inquiry Lesson experimentation what factors affect the dissolving rate of alka-seltzer and which do not have any affect. They will pose questions in their mind, and answer these through their findings. 5 Dissolving Rate Inquiry Lesson 6 Dissolving Rate Inquiry Lesson Lesson Plan 2. (a) List several objectives you have for students (both process and content). *The student will be able to understand the concepts concerning dissolving rate. *The student will be able to identify the variables that affect the dissolving rate. (mass/volume of substance, concentration, movement, temperature, surface area, matter being dissolved) *The student will be able to identify that the rate of a chemical reaction is affected by the physical size of the reactants. *The student will be able to predict which concept has the most affect on the dissolving rate. *The student will be able to construct a graph using their observations and measurements through out the investigation. *The student will be able to define concentration. *The student will be able to define dissolve. *The student will be able to define solute. *The student will be able to define solvent. *The student will be able to define surface area. *The student will be able to define kinetic energy. 7 Dissolving Rate Inquiry Lesson *The student will be able to define matter. *The student will be able to define molecule. *The student will be able to define mass. *The student will be able to define volume. *The student will be able to define reactant. *The student will be able to demonstrate that the rate of a chemical reaction can be increased by increasing the temperature of reactants. *The student will be able to observe the factors that affect the dissolving rate. *The student will be able to measure which factor most affects the dissolving rate. *The student will be able to classify their observations in order from most powerful to least powerful affect on the dissolving rate. *The student will be able to interpret their constructed graph and communicate their discoveries to their peers. *The student will be able to name all 6 of the given factors that affect the dissolving rate. *The student will be able to determine that increased surface area increases the rate of dissolving. *The student will be able to determine that the dissolving rate is disturbed by movement. *The student will be able to determine that the dissolving rate is changed by temperature. 8 Dissolving Rate Inquiry Lesson *The student will be able to determine that the dissolving rate is affected by surface area. *The student will be able to determine that the dissolving rate is altered by the matter being resolved. *The student will be able to determine that the dissolving rate is influenced by the mass or volume of the substance. 2. (b) What questions might students have about the concept(s)? What questions could you ask students during the course of the lesson? Student Questions: 1. Does the temperature of the water affect the dissolving rate? 2. Does the surface are of the water affect the dissolving rate? 4. Does the concentration of solvents affect the dissolving rate? 5. Are dissolving rate and reaction rate the same? 6. Why do different factors affect the dissolving rate differently? 7. Which variable will affect the reaction rate the most? 8. Which factor will affect the reaction rate the least? 9. Will mixing these chemicals together cause an explosion? 10. Why do we need to understand the characteristics of a dissolving rate? Teacher Questions: 9 Dissolving Rate Inquiry Lesson 1. In 8 ounces of water, how long would it take 1 alka seltzer tablet to melt? 2. What would happen to the reaction rate if the temperature is doubled? 3. What would happen to the reaction rate if you used hot tap water? 4. What happens to the surface area as the particle size decreases? 5. Does particle size or temperature have more of an affect on the rate of reaction? 6. What occurs when the concentration of the reactants decreases? 7. Does stirring have any affect on the rate of reaction if the reacting substances are already in the solution? 8. What happens when two substances react together? 9. Define the terms solute, solvent, concentration, and reactant. 10. Classify from most powerful to least powerful the effectiveness of each variable investigated on the dissolving or reaction rate. 11. Interpret and demonstrate the graph you constructed to your classmates during a class discussion about your investigation discoveries. 12. Compare and contrast the effects of the mass/volume of a substance, concentration, movement, temperature, surface area, and matter being dissolved on the dissolving or reaction rate. 13. Construct a graph using one component that affects the dissolving rate and a time component. 10 Dissolving Rate Inquiry Lesson 14. Predict which of the factors to be investigated will have the most affect on the dissolving rate. 15. Describe the characteristics of a solute. 16. Identify the solvent used in each investigation. 2. (c) What is the teacher’s role during the lesson? The teacher acts as the facilitator during these investigations. The teacher will challenge the students to further experiment by questioning their processes and asking questions to guide them through their investigation. Many students may at times feel “stuck.” The teacher may use these opportunities to ask preplanned questions to advance the focus of their exploration. The teacher’s role is to pose the central question of the investigation which is “What affects the dissolving rate?” The teacher’s questioning helps students stay interested in the investigation. The teacher will use questions, discussions, and assigned tasks to evaluate the students progress during the investigation and their full understanding at the conclusion of the investigation. During the explain phase, the teacher plays a more active role than in the explore phase. The teacher does not give away answers or directly answer questions but returns questions with questions. During this phase, the teacher takes the opportunity to make sure the students have an understanding of the concept at hand. During the expand phase, the teacher’s role is to bring up realistic connections between the investigation and a real life situation. An example would be: with the alka seltzer investigation, the student will not only have a process understanding of “dissolving rate” but will also have an understanding of how the medicine “alka seltzer” helps people feel better. The teacher also uses the opportunity to make sure the 3 H’s are being addressed, that the head, heart, and hands are being attended to. While evaluating through out the 11 Dissolving Rate Inquiry Lesson investigation, the teacher’s role includes testing the given concepts outside of the typical confines of the assignment, question, and identify any misconceptions. 2. (d) What data will be collected by students and how will it be collected? The students will collect data through out the divisions of the investigation. The students will time dissolving rates, chart temperatures, administer and observe the affects of movement, measure particle size, surface area, concentration, and mass and volume of a given substance. 2. (e) How will the data be used/represented by students? The students will use the data collected to construct a graph with multiple components. The students will use the graph to deepen their understanding of dissolving rates. They will also use the graph to share and discuss their findings with their classmates. The graphs will also be used as a form of evaluation for the teacher to make sure they understand the concepts and that there are not misconceptions. The 4-E Learning Cycle: EXPLORE During the explore phase, the students will perform 4 experiments that will each add to their understanding of dissolving rate. Each investigation will be centered around the use of alka seltzer. The questions for each investigation are as follows: 1. How does temperature affect the dissolving rate? The idea: In order for a chemical reaction to occur, the particles, atoms or ions, which are reactants, must physically come into contact with one another. Anything that increases the 12 Dissolving Rate Inquiry Lesson frequency of these encounters will increase the rate at which the products are formed. The rate of chemical reaction can be increased by increasing the temperature of these reactants. 2. How does particle size affect the dissolving rate? The idea: The rate of a chemical reaction is affected by the physical size of the reactants. Decreasing the size of the particles which make up a given weight will increase the number of particles represented by the same weight. Smaller particle size results in an increase in the rate of reaction because the surface area of the reactant has been increased. 3. How does concentration affect the reactants? The idea: The rate of chemical reaction depends on the frequency of the collisions between the atoms or ions of the reactants. As the concentrations of the reactants decreases, the frequency of collisions decreases, and the rate of reactions slows down. 4. What factors affect the rate of chemical reactions? The idea: If any of the products or reactants involved in a chemical reaction are gases, the rate of reaction will decrease as pressure on the system is increased. The purpose of this phase is to get students interested in the concept and motivated to ask their own questions about the investigation or factors of it. The students are encouraged to think on their own, and the experiments provide the student an opportunity to be introduced to the idea of dissolving rates. In this phase, students will be testing their ideas and constructing knowledge about a new concept. EXPLAIN 13 Dissolving Rate Inquiry Lesson During this phase the students will be asked to define concentration, dissolve, solute, solvent, surface area, kinetic energy, matter, molecule, mass, volume, and reactant. These words will give the students the necessary terminology to better understand the concept. The teacher at this point will use the concept map to link all of the variables together in understanding the multiple characteristics and variables of the dissolving rate. This phase will better explain the explore phase, and at this point, the students will be “hooked” and more apt to give their attention to the subject and understanding it better. During this phase, the students will compile data and collaborate with their peers to discuss their findings. Hopefully, during this phase, the student will being to see the concept as a whole, and not as multiple divisions of experiments. EXPAND During the expand phase there are multiple arenas to explore. One experiment teachers could add is called “Alka Rockets”. In this experiment, the student will design a paper rocket propelled by alka seltzer and water to demonstrate Newton’s Third Law of Motion. This experiment could be tied into social studies by incorporating a unit on events in space. This experiment could be the science component in a social studies unit, or an art component in a social studies unit. In the lesson centered around dissolving rate, the teacher can incorporate math by simply adding graphing as a component of the investigation, as we have done. To accommodate ELL students, the teacher could create a matching game using index cards and the needed vocabulary terms to help the students get more familiar with the terms which will lead to a better understanding of the concept. Accommodating special needs students covers a very wide array of issues. Without knowing the specific needs of the student, or having seen an I.E.P, we can only give broad examples of accommodation. If the student has a physical constraint, the teacher could pair them with a student whom they trust to help the special needs student by both performing the actual 14 Dissolving Rate Inquiry Lesson tasks of investigation, but still including the student while discussing the concept or forming opinions and understanding about the subject. If the student has a mental disability, they can also be paired with a partner, but may be asked to participate in the investigation as mush as possible, and may not be held accountable for questions that are at a generally high cognitive level. Teachers may also consider finding web simulations or pre recorded simulations to allow the special needs student to view in a quieter environment for better understanding. The expansion phase is used to build on a given concept, use the concepts in a different way, and give students a real world application of the concept. EVALUATE Evaluation will occur through out the investigation via the teacher asking multiple challenging questions to encourage a deeper understanding of the concept. The teacher may also use the graph(s) constructed during the investigations as evaluations to determine the level of understanding of each student. And the teacher may also use class discussions to gage the understanding of each student as well as each individual’s response to purposed questions through out the investigation. 15