electrostatic repulsion and hybridization
advertisement

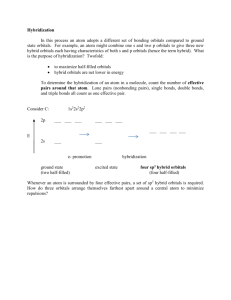
ELECTROSTATIC REPULSION AND HYBRIDIZATION Data have shown that bond angles for atoms in molecules with p orbitals in the outer shell do not conform to the expected 90° separation of an x, y, and z axis orientation. This variation can be expressed by electrostatic repulsion between valence electron charge clouds or by the concept of hybridization. Electrostatic repulsion uses as its basis the fact that like charges will orient themselves in such a way as to diminish the repulsion between them. 1. Mutual repulsion of two electron clouds forces them to the opposite sides of a sphere. This is called a linear arrangement. 2. Minimum repulsion between three electron pairs occurs when each is at the vertices of an equilateral triangle inscribed in a sphere. This is called trigonal planar. 3. Four electron pairs are farthest apart at the vertices of a tetrahedron inscribed in a sphere. This is called a tetrahedral shape. 4. Mutual repulsion of six identical electron clouds directs them to the corners of an inscribed regular octahedron. This is said to have octahedral geometry. These same configurations can also be arrived at through the concept of hybridization. Briefly stated, this means that two or more pure atomic orbitals (usually s, p, and d) can be mixed to form two or more hybrid atomic orbitals that are identical. This can be illustrated as follows: 1. sp Hybrid Orbitals Beryllium fluoride spectroscopic measurements reveal a bond angle of 180° and equal bond lengths. To accommodate the experimental data we theorize that a 2s electron is excited to a 2p orbital; then the two orbitals hybridize to yield two identical orbitals called sp orbitals. Each contains one electron but is capable of holding two electrons. 2. sp Hybrid Orbitals Boron trifluoride has bond angles of 120° of equal strength. To accommodate these data the boron atom hybridizes from its ground state of 1s22s22pl to: 3. sp3 Hybrid Orbitals Methane, CH4, can be used to illustrate this hybridization. Carbon has aground state of 1s22s22p2. One 2s electron is excited to a 2p orbital, and the four involved orbitals then form four new identical sp3 orbitals. In some compounds where only certain sp3 orbitals are involved in bonding, distortion in the bond angle occurs because of unbonded (unshared) electron repulsion. Examples: a. Water, H2O. b. Ammonia, NH3 4. spd2 Hybrid Orbitals These orbitals are formed from the hybridization of an s and a p electron promoted to d orbitals and transformed into six equal spd2 orbitals. The spatial form is: