PEXIVAS: The Largest Randomised Controlled Trial in Vasculitis to
advertisement

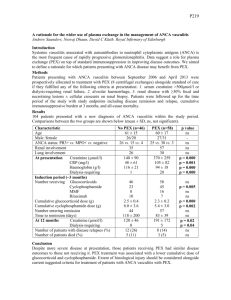
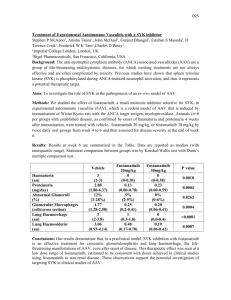
P259 PEXIVAS: DESIGN OF A RANDOMISED CONTROLLED TRIAL OF PLASMA EXCHANGE AND GLUCOCORTICOID DOSING IN SEVERE ANCA ASSOCIATED VASCULITIS Casian, A1, Merkel, P2, Walsh, M3, Jayne, D1 1 Renal Unit, Addenbrooke’s Hospital, Cambridge, 2Boston University, USA, 3McMaster University, Canada INTRODUCTION: The current outcomes of ANCA associated vasculitis (AAV) are frequently poor for those presenting with severe renal disease or pulmonary haemorrhage and drug-related toxicity contributes to mortality and morbidity. Patient and renal survival at 5 years is only 54% for those presenting with an eGFR<50 mls/min, and severe pulmonary haemorrhage has a mortality of 50% in the first year. Plasma exchange (PLEX) has been shown in the recent MEPEX trial, and in a meta-analysis of all PLEX trials in vasculitis, to improve renal recovery in severe renal vasculitis but it is unclear whether this benefit is sustained and whether PLEX reduces mortality or is effective in pulmonary haemorrhage. The expense and potential complications of PLEX demand a stronger evidence base before its use can be routinely recommended. A scientific rationale for PLEX has emerged with demonstration of the pathogenicity of ANCA but questions remain as to its precise mechanism of action, optimal dosing and combination with concomitant drug therapy. Current glucocorticoid (GC) dosing is a major contributor to severe adverse events, a particular concern in those with uraemia or respiratory failure. Recent optimisation of cyclophosphamide regimens and demonstration of the efficacy of alternative therapy with rituximab has re-focused attention on whether GC dosing can be safely reduced without compromising efficacy. PRIMARY OBJECTIVES: 1) To determine the efficacy of PLEX in addition to immunosuppressive therapy and GC at reducing death and end-stage renal disease (ESRD), 2) To determine whether a reduced dose GC regimen is non-inferior to a standard dose GC regimen with respect to death and ESRD, as well as being superior with respect to adverse events (especially infections). Secondary and Exploratory Objectives include the effects of the two interventions on disease activity, quality of life, cost-effectiveness, disease-related damage, long-term renal function, vasculitis biomarkers. DESIGN: 500 Patients with new or relapsing severe AAV (renal vasculitis with recent eGFR< 50 mls/min or/and pulmonary haemorrhage), >=15 years of age, from 60 centres in 15 countries will be recruited over 5 years and followed-up for a maximum of 7, minimum 2 years. Those unable to consent due to severe AAV can be enrolled provided that consent will be obtained from the patient’s legal representative. Randomisation will be performed by a central computerised facility at the Birmingham Clinical Trials Unit. PEXIVAS is an open label RCT with a two by two factorial design, testing PLEX versus no PLEX and a standard dose GC regimen versus a reduced dose GC regimen. The 500 patients will be divided into 4 groups with 125 in each, receiving treatment as follows: 1) PLEX + standard dose GC 2) PLEX+ reduced dose GC 3) no PLEX + standard dose GC 4) no PLEX + reduced dose GC. All patients will receive induction therapy, usually cyclophosphamide, with rituximab as a permitted alternative and maintenance therapy with azathioprine. Immunosuppressive and GC dosing will be determined by the protocol for the first 12 months after trial entry. CONCLUSIONS: PEXIVAS aims to clearly determine both the role of PLEX in severe AAV and whether GC dosing can be safely reduced. The results will be of potential benefit to other vasculitis presentations and the use of PLEX in other immune-mediated disorders. The collaborative nature of the trial, stretching over three continents will promote parallel vasculitis research and the wider introduction of best practice in vasculitis management.