Abstract
advertisement

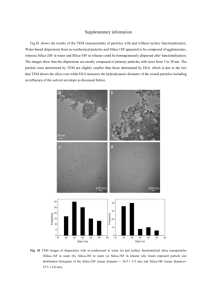
Summer Program Report Prepartion of Silica and Titanium Oxide Particles by Microwave Method Han-Lang Wu Advisor: Prof. Chen-Chi M. Ma D3, Chemical Engineering, National Tsing-Hua University 2006, Jul. 3 to Sep. 1 stay in Japan Advisor in Japan: Prof. Yoshiki Chujo Department of Polymer Chemistry, Kyoto University mail: Mobile: Tel: Fax: d913650@oz.nthu.edu.tw +886 929103102 +886 3 5715131 ext 33694 +886 3 5725924 1 Introduction This is the report of my summer study program in Japan. There are two parts in this report, which are Experiments and Life & Experiences. I (Han-Lang Wu) am a third year PhD graduate student of National Tsing-Hua University. My advisor is Prof. Chen-Chi M. Ma. My major is chemical engineering. I am interested in polymer chemistry, polymer physics, polymer composites, hybrids and proton exchange membrane for fuel cell. Because of this summer program, I have a chance to visit Prof. Chujo’s laboratory, Department of Polymer Chemistry, graduate school of engineering, Katsura campus, Kyoto University. Current research fields of Prof. Chujo’s laboratory focus on the development of polymerization reaction, reactive polymer, environmentally responsible polymer, intelligent polymer, organic-inorganic polymer hybrid, metal nanoparticle, and template mineralization on the basis of synthetic organic chemistry as well as inorganic chemistry. There are two other faculties, associate professor, Kensuke Naka, and assistant professor, Yasuhiro Morisaki in Prof. Chujo’s laboratory. I stayed in Prof. Chujo’s laboratory from July 3 to September 1. In this study, microwave was utilized to synthesize spherical particles, which can be prepared rapidly with uniform sizes. The SiO2 particles were firstly synthesized following the synthesis with TiO2 precursor in order to form core-shell structure. Generally, the microwave method is fast, simple, and highly energy efficient. In Prof. Chujo’s lab they have been used microwave-assisted sol-get method to synthesis hybrid materials from an organic polymer and a sol-gel precursor. Recently, the microwave method has been applied to the preparation of metal oxide nanoparticles. For example, using a microwave assisted polyol method, TiO2 nanocrystallites, SnO2 sols and BaTiO3, 2 Ba6Ti17O40, BaZrO3, PbTiO3 nanoparticles have been prepared.1-6 Among the inorganic particles, TiO2 and its related materials are widely investigated due to its widespread applications in photovoltaic cells, batteries, separations, sensing, optical emissions, photonic crystals, catalysis and photo-catalysis, selective adsorption, ion exchange, ultraviolet blockers, smart surface coatings, and as functional filling materials in textile, paints, paper, and cosmetics. Experiments Synthesis of silica particles by microwave method Adachi et al. have been firstly proposed synthesis of sub micrometer silica spheres in non-alcoholic solvent by microwave-assisted sol-gel method.7 The synthesis procedure is shown below: 40 ml bis(2-methoxymethyl)ether (diglyme), 1 ml tetramethoxysilane (TMOS) and 0.5 ml 0.1 M HCl solution were added into 50 ml sample bottle. The mixture was stirred for 1 hour at room temperature. The solution was poured into 50 ml Teflon beaker. The beaker was put at the center of the microwave oven and the irradiation power and time. To get the most uniform silica sphere, the irradiation power is 1000 W for 3 minutes. After being microwave irradiated, the Teflon beaker was taken out of the microwave oven and cooled down at room temperature. The white solution was precipitated by centrifuging with 2500 rpm for 30 minutes. The upper solution was removed and the white product was washed by tetrahydrofuran (THF). The centrifuge tube was capped with tissue paper and the product was dried under vacuum at 60 oC. Fig. 1 is the SEM images of the silica particles by microwave method. Comparing to the conventional heating method, the silica particles by microwave method are less 3 aggregated and their surfaces are fairly flat. Surprisingly, the diameter of these silica particles is uniform. The diameter of silica particles is depends on the power and the time of microwave processing. The input energy of Fig. 1(a) and (b) is 180 kJ and 120 KJ, respectively. The higher the input energy, the larger silica particles can be derived. The average particle size in Fig. 1(a) is 700 nm, while is 400 nm in Fig. 1(b). The growth of silica particles by microwave method was assumed as the following steps. Firstly, the silica precursors, TMOS, were reacted to form oligomer during the stirring at ambient temperature. The oligomer can be regarded as silica seeds. When these silica seeds were irradiated in the microwave, the energy of microwave was absorbed at the surface of these slilca seeds directly because of the presence of polarized silanol groups. The elevated surface energy causes the enhanced reactivity on the surface of silica seeds that makes TMOS reacted rapidly on it. On the other hand, the non-alcoholic solvent absorbs lower irradiation energy, so that the solution temperature is low comparing to that of the surface of silica particles. The temperature difference leads to the greatly different reactivity between the surface of silica particles and the solution hence the uniform silica particle sizes were derived. However, in the conventional heating the temperature was uniform in whole environment, so that the energy is transferred slowly through the vibration of the solvent molecules and the silica particle formation is slow. In such case, the silica seeds grow irregularly and the aggregation is easily occurred. Synthesis of silica-titanium oxide core-shell particles The procedure of silica-titanium oxide core-shell particles is described as the followings. The silica particles are re-dispersed in 50 mL diglyme. 0.5 mL 1M base 4 solution (tributylamine) and 1 mL titanium tetraisopropoxide (Ti(OPri)4 or TTIP, 99.999%), which is the precursor of the TiO2, were added into the solution. Tertiary amines or quaternary ammonium hydroxides were employed as catalysts for polycondensation in order to ensure a crystalline product. The mixture was stirred at room temperature for one hour and was further microwave irradiated for 3 minutes with the power of 1000 W. Cozolli et al have been found that the easy crystallization process in the mild conditions (at as low a temperature as 80 °C), which can be attributed to a specific action played by bulky amines and alkylammonium hydroxides during the synthesis.8 They suggested that amines could promote a chemical reversibility in the Ti-O bonding formation. The amine/alkoxy or amine/titanyl exchange can occur when the alkoxide precursors of a partially formed titania network attack the electrophilic titanium center, therefore, a Ti-O bond breaking and forming. The SEM images of silica/titanium oxide particles were shown in Fig. 2. Instead of the core-shell structure, the TiO2 precursors form many cubic structures. As the increase of irradiation time and input power, those TiO2 cubic particles grow larger and cover the whole surface of silica particles. The reactivity of silanol is quite lower than that of titanium tetraisopropoxide, hence the TTIP reacted with itself instead of silanol thus the formation of TiO2 cubic instead of core-shell structure. At room temperature, TTIP solution turns from transparent liquid to white solid in short times. Even quickly inject TTIP into diglyme, the small and transparent particles can be found in the solution. Besides, the crystalline of TiO2 restricts the formation of sphere shape, i.e. growing randomly. The crystalline can be investigated via X-ray diffraction. 5 Conclusion In this study, the silica particles with uniform diameter have been synthesized by microwave-assisted sol-gel method. Effects of the input power and the irradiated time have been discussed. In this stage, the core-shell silica-titanium oxide structure has not been synthesized successfully, but it is the good beginning to study this issue. References 1. W. Wang, B. H. Gu, L. Y. Liang, and W. A. Hamilton, J. Phys. Chem. B, 107, 12113 (2003) 2. J. E. G. J. Wijnhoven and W. L. Vos, Science, 281, 802 (1998) 3. P. Jiang, J. F. Bertone, and V. L. Colvin, Science, 291, 453 (2001) 4. O. D. Velev and E. W. Kaler, Adv. Mater., 12, 531 (2000) 5. Y. N. Xia, B. Gates, and Z. Y. Li, Adv. Mater., 13, 409 (2001). 6. W. Stober and A. Fink, J. Colloid Interface Sci., 26, 62 (1968). 7. K. Adachi, T. Iwamura, Y. Chujo, Chem. Lett., 33, 1504 (2004) 8. P. D. Cozzoli, A. Kornowski, H. Weller, J/ Am. Chem. Soc., 125, 14539. (2003) 9. Chemseddine, A.; Moritz, A. Eur. J. Inorg. Chem., 235. (1999) 10. Bischoff, B. L.; Anderson, M. A. Chem. Mater., 7, 2. (1995) 11. Murakami, Y.; Matsumoto, T.; Takasu, Y. J. Phys. Chem. B, 103, 1836. (1999) 12. Poznyak, S. K.; Talapin, D. V.; Kulak, A. I. J. Phys. Chem. B, 105, 4816. (2001) 6 (a) 1000 W, 3 minutes (b) 500 W, 4 minutes Fig. 1. The silica particle by microwave irradiation. 7 (a) 500 W, 4 minutes (b) 1000 W, 3 minutes Fig. 2. The silica/titanium oxide particles by microwave method. 8 Life & Experiences This is my first time to visit Japan and also my longest time for me to stay abroad that I have ever been. Japan is really a clean and fine country. People work very hard at there. Japanese students are very cooperative, they like to work together. In Japan, there are three teachers in one laboratory. Generally, they are professor, associate professor and assistance professor. In Prof. Chujo’s laboratory, they are around 20 graduate students; the ratio of teacher to student is high, so that students at there can get more time to discuss with their teachers. The facilities in Prof. Chujo’s laboratory are very good. They have nuclear magnetic resonance (NMR), gel permeation chromatography (GPC), mass-gas chromatography (GC-mass), scanning probe microscopy (SPM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), polymer crystalline measurements, infrared spectroscopy (IR), differential scanning calorimeter (DSC), etc. In Taiwan, one laboratory could not have so many equipment. It is very suitable for doing experiments in Japan. In addition, the Katsura campus of Kyoto University is very beautiful. This campus is on the mountainside, one can view the whole Kyoto city in Katsura campus. The night view of Kyoto city is also very nice. The landmark in Katsura campus is its clock tower. This clock tower has LED display, which has high brightness. The meals in the student restaurant are delicious. There are so many dishes such as pork, beef, chicken, fish, noodles, etc. The environment of restaurant is also good. 9 Because Japanese food and Taiwanese food are not so different, there is no problem for me to get use to Japanese food. Acknowledgement So many people and organizations I want to acknowledge. First of all, I would like to thank Interchange Association Japan (IAJ) and National Science Council (NSC) for their financial support. Thanks to my advisor, Prof. Ma, for allowing me to stay two months in Japan. I also want to thank Ms. Tsunoda-san and Ms. Yokoi-san for their kindly help. Still, I would like to appreciate Kokado-san for the assistance on my experiments. A lot of thanks are giving to Wada-san, Inagi-san, Ouchi-san and Nakahashi-san for taking me to many interesting places. I really have fun with all of you. Thank you, Nakamura-san, for teaching me Japanese, taking me home after mini soccer games, playing table tennis with me. I would also like to acknowledge Saito-san for introducing such beautiful city, Kobe, to me. Furthermore, I would like to thank Fujita-san for many pleasant talking. Special thanks are giving to Huang-san and Lin-san. I feel comfortable to stay in this foreign place, Japan, because I can talk with you in Chinese. In addition, I am appreciated Dr. Lee of MRL, ITRI for introducing me to Prof. Chujo, so that I can have these wonderful days in Japan. Last but not the least, I would like to dedicate much thanks to Prof. Chujo for giving me so nice research environment and thoughtful care. I really learned and enjoyed a lot, thank you very much. 10

![LAB 4 FB Safety [BH]](http://s3.studylib.net/store/data/007109339_1-10edf2f99cf9e3f5eb5770ce96c065cf-300x300.png)