Supporting information Fabrication of teardrop
advertisement

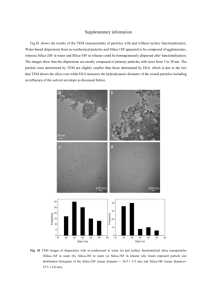
Supporting information Fabrication of teardrop-shaped silica particles in polyelectrolyte diluted solution through in situ sol-gel process Song LinYong*, Lin ChunLei, Wang Lan, Sheng HaiLin, Zhou YiFeng, Nie WangYan The Key Laboratory of Environment-friendly Polymer Materials of Anhui Province, College of Chemistry & Chemical Engineering, Anhui University, Anhui 230039, P. R. China. Figure 1 TEM and SEM of silica particles using 2.25mg NaPAA Figure 2 The photograph of silica particles at different NaCl concentration (From left to right, A, B, C, and D). (recipe:Water 15mL, Ethanol 35mL, TEOS 1mL, NH3H2O 1mL) With the increasing of NaPAA concentration, the solution’s clarity varied from transparent to subtransparent, which indicated that the average diameter of silica particles growth slightly. And this result is agreed with the TEM and SEM images. With the creasing of salt concentration, the colloidal stability shows an obviously falling. Figure 3 The TEM images of silica particles at different reaction time. (A) 1 hour, (B) 1.5 hour, (C) 3 hour, (D) 8 hour. (recipe: NaPAA 1.5mg, Water 15mL, Ethanol 35mL, TEOS 1mL, NH3H2O 1mL) IR spectra were recorded on a Bruker Vector-22 Fourier transform infrared (FT–IR) spectrometer. The samples were grounded with KBr crystal and the mixture of them was pressed into a flake for IR measurement. Figure 4. FT-IR spectrum of the teardrop-shaped silica particles The FTIR spectrum on the washed samples shows that three fundamental vibration bands corresponding to Si-O-Si asymmetric stretching, symmetric stretching, and bending vibrations appear at near 1102, 800, and 475 cm-1, respectively. The peak at 952cm-1 belongs to the Si-O stretch vibration of Si-OH bond. All of the main bands in the FTIR spectrum were attributed to silica, which approved the complete removal of inside colloid aggregates.




![LAB 4 FB Safety [BH]](http://s3.studylib.net/store/data/007109339_1-10edf2f99cf9e3f5eb5770ce96c065cf-300x300.png)

![[Supporting Information] Optical properties of noncontinuous gold](http://s3.studylib.net/store/data/007684451_2-c04229bef8743b93daf5996a3836829b-300x300.png)



