revised sample lab report
advertisement
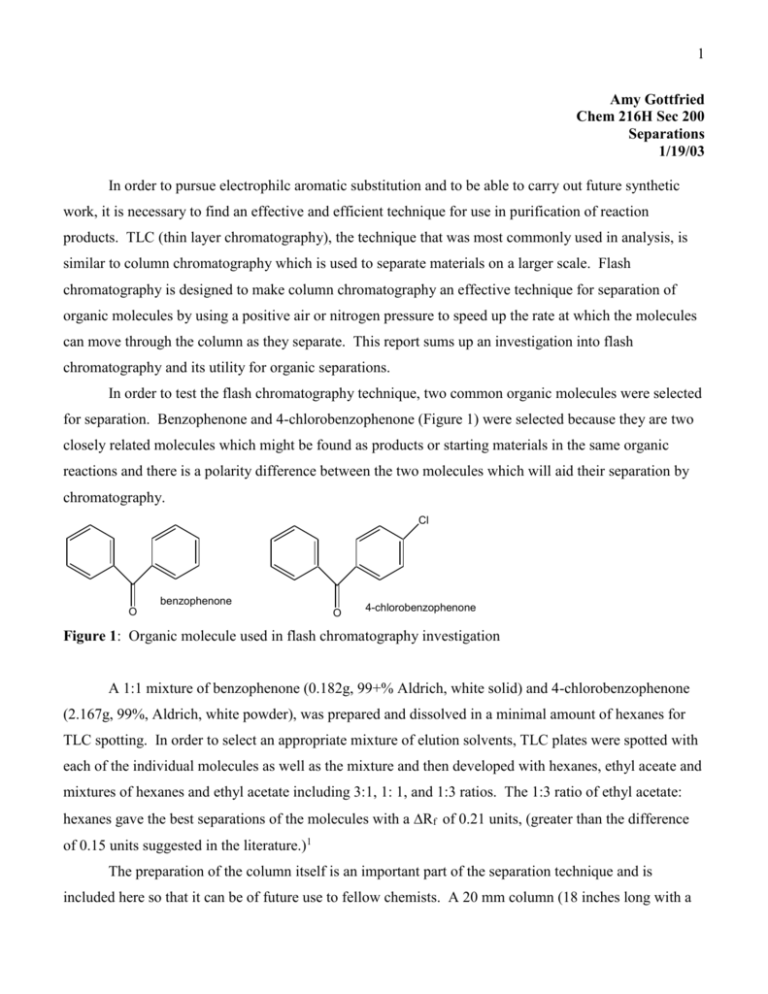
1 Amy Gottfried Chem 216H Sec 200 Separations 1/19/03 In order to pursue electrophilc aromatic substitution and to be able to carry out future synthetic work, it is necessary to find an effective and efficient technique for use in purification of reaction products. TLC (thin layer chromatography), the technique that was most commonly used in analysis, is similar to column chromatography which is used to separate materials on a larger scale. Flash chromatography is designed to make column chromatography an effective technique for separation of organic molecules by using a positive air or nitrogen pressure to speed up the rate at which the molecules can move through the column as they separate. This report sums up an investigation into flash chromatography and its utility for organic separations. In order to test the flash chromatography technique, two common organic molecules were selected for separation. Benzophenone and 4-chlorobenzophenone (Figure 1) were selected because they are two closely related molecules which might be found as products or starting materials in the same organic reactions and there is a polarity difference between the two molecules which will aid their separation by chromatography. Cl benzophenone O O 4-chlorobenzophenone Figure 1: Organic molecule used in flash chromatography investigation A 1:1 mixture of benzophenone (0.182g, 99+% Aldrich, white solid) and 4-chlorobenzophenone (2.167g, 99%, Aldrich, white powder), was prepared and dissolved in a minimal amount of hexanes for TLC spotting. In order to select an appropriate mixture of elution solvents, TLC plates were spotted with each of the individual molecules as well as the mixture and then developed with hexanes, ethyl aceate and mixtures of hexanes and ethyl acetate including 3:1, 1: 1, and 1:3 ratios. The 1:3 ratio of ethyl acetate: hexanes gave the best separations of the molecules with a Rf of 0.21 units, (greater than the difference of 0.15 units suggested in the literature.)1 The preparation of the column itself is an important part of the separation technique and is included here so that it can be of future use to fellow chemists. A 20 mm column (18 inches long with a 2 Teflon stopcock and a 24/40 female joint at the top) was packed from the bottom with a small plug of glass wool, a 0.5 cm layer of sand, and a layer of silica gel (Aldrich, 70-270 mesh) approximately six high. The column (stopcock open) was tapped with a cork ring to pack the silica gel before topping it with 0.5 cm of sand. The column was then filled with the separation solvent (1:3 ethyl acetate:hexanes) and capped with a 24/40 male cap containing two 4mm tubes. One of the tubes was capped with a septum for use as a pressure relief valve while the second was attached to an air jet via tygon tubing containig a clamp for controlling air flow. The eluent was forced through the silica gel to force out any air and pack the column using positive air pressure at the top of the column. A flow rate of ~ 2 cm of eluent per minute was established by adjusting the clamp while the column was under pressure. (Note: as with any column, the silica gel should not be allowed to run dry! If necessary, eluent can be added by releasing the pressure.) Once the column was packed satisfactorily, the eluent level was lowered to the top of the sand. A 20% solution of the 1:1 mix of the two compounds in 1:3 ethyl acetate:hexanes was added to the column followed by a rinsing of the sides of the column with ~5 mL of the solvent mixture. The compounds were forced onto the silica gel using pressure to lower the eluent level to the top of the sand and finally, the column was filled with eluent. Pressure was applied for a flow rate of ~2 cm per minute and 10 mL fractions were collected from the column until the eluent reached the top of the sand. Each fraction was spotted on a TLC plate which was run with the same 1:3 solvent mixture used for the column. The first product, benzophenone (Rf = 0.61) was found in fractions 4-8, while the 4chlorobenzophenone (Rf = 0.39) was found in the last two fractions (19 and 20.) Eluent was added to the column to extract the remainder of the 4-chlorobenzophenone. Ten more 10 mL fractions were taken and the 4-cholorbenzophenone was found on in the first three of these. The fractions containing the each of desired products were combined into two tared flasks and solvent removed via rotovap. Each product was weighed and identified by melting points and 1H NMR (Tables 1 & 2.) Table 1: Data for Benzophenone Original Separated 0.182 0.175 48-49 47-48 Shift JH(Hz) Shift JH(Hz) (ppm) (ppm) 7.70 d 2H 6.4 7.71 d 2H 6.3 7.17 t 1H 14.4 7.19 t 1H 14.4 7.01 t 2H 10.8 6.99 t 2H 10.9 a1 H NMR (200MHz, 25oC) CDCl3 b NMR spectra attached. Figure 2: 1H NMR original benzophenone. Figure 3: 1H NMR of the separated benzophenone. Wt(g) mp (oC) NMRa,b 3 Table 2: Data for 4-Chlorobenzophenone Original Separated 0.167 0.158 Wt(g) o 77-78 mp( C) 76-77 a,b Shift JH(Hz) Shift JH(Hz) NMR (ppm) (ppm) 7.70 d 2H 6.4 7.71 d 2H 6.3 7.42 d 2H 8.4 7.42 d 2H 8.4 7.17 t 1H 14.4 7.19 t 1H 14.4 7.07 d 2H 8.4 7.07 d 2H 8.4 7.01 t 2H 10.8 6.99 t 2H 10.9 a1 o H NMR (200MHz, 25 C) CDCl3 b NMR spectra attached. Figure 4: 1H NMR original 4-chlorobenzophenone. Figure 5: 1H NMR of the separated 4-chlorobenzophenone. The data in Tables 1 and 2 shows that a 1:1 mixture of the two molecules benzophenone and 4chlorobenzophenone were cleanly separated by flash chromatography using an eluent of 1:3 ethyl acetate:hexanes. The melting points of both the original and separated compounds are within experimental error (1oC) and the 1H NMR spectra of the two separated compounds contain only those compounds and correspond directly to the two original compounds that were mixed together. Both benzophenone and 4-chlorobenzopheneone were almost completely recovered after the separation (96% and 95% yield.) In conclusion, flash chromatography is a technique that is both effective and efficient at cleanly separating closely related organic molecules without significant loss of material. Flash chromatography is most effective when a solvent is chosen to achieve maximum separation with high Rf values. The literature should be consulted when choosing flow rate, column size, fraction size, and column loading in order to achieve optimal results.2 Attachments: Figure 2: Figure 3: Figure 4: Figure 5: 1 H NMR original benzophenone H NMR of the separated benzophenone. 1 H NMR original 4-chlorobenzophenone. 1 H NMR of the separated 4-chlorobenzophenone. 1 Each spectrum is labeled with the figure title, and a sketch of the molecule with all of the peaks assigned to the proper proton. For example: 4 H H b Hc b Ha H Hc a H H b b Ha O Ha The doublet at 7.70 ppm would be labeled “a,” the triplet at 7.17 ppm labeled “c,” and the triplet at 7.01 ppm labeled “b.” A couple of other notes: 1) For the compounds benzophenone and 4-chlorobenzophenone, UV-Vis and IR would not have been particularly helpful in distinguishing between the two compounds that were separated. In the case of an aromatic vs. non-aromatic, UV-Vis would have been more helpful and certainly been appropriate to include. 2) The data contained within this lab report is based on reasonable assumptions and not actual experimentation so the Rf values, melting points, chemical shifts, columns size, etc. are not necessarily the same as those recorded in your lab notebook 3) This report is just a guideline, not an absolute mandate for how to prepare your reports. 4) It is much easier for the GSI’s to grade if you use spacing such as 1 ½ or double rather than single spacing. 5) Page numbers are always useful 6) The list of attachments may help you to organize your spectra and will not count towards your 3 pages of report 7) Please use references and make sure that you cite them properly 8) This report contained more experimental and less analysis than many of the future ones will. This one also did not contain any reaction schemes. 1 2 http://www.saiadsorbents.com/flash.htm (accessed Jan 19, 2003) Still, W. C., Kahn, M., Mitra, A. J. Org. Chem. 1978, 43, 2923.






