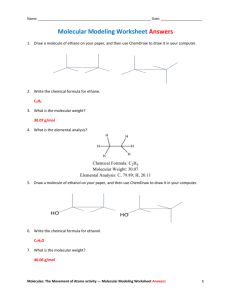
Molecular models can be very helpful for visualizing the three
advertisement

CHEM 350 Principles of Organic Chemistry I Lab Prof. T. Nalli, WSU Expt #2 - Molecular Modeling and Properties of Alkanes and Alkyl Halides Relevant Reading - Bruice, Chapter 2.9, 2.11 Overview Molecular models can be very helpful for visualizing the three-dimensional nature of organic structures. The models can be physically manipulated (for example, bonds can be rotated), thus mimicking the types of dynamic processes the actual molecules undergo. There are many different types of molecular model kits (including computer software) and each has its own particular weaknesses and strengths. Dreiding skeletal models are very precise in modeling the actual bond angles and bond lengths of simple organic molecules and the single bonds rotate freely just the same as in real molecules. Space-filling models accurately reflect the amount of space the atoms occupy at the expense of not being able to see the bonds very well. In contrast, ball and stick models show the bonds in a molecule quite clearly by exaggerating their lengths and understating the sizes of the atoms. In most model kits and programs, black is used to represent carbon with red reserved for oxygen, blue for nitrogen, green for chlorine, and white for hydrogen. The Prentice Hall molecular modeling kit can be used to make both space-filling models and ball and stick models (see kit instructions). The bond angles in models made using this kit are not necessarily very accurate (especially those around trigonal planar atoms), but the bonds are easily rotated and bent thus allowing great flexibility in the number and type of molecules that can be represented. Computer molecular modeling programs such as Chem3D are generally capable of rendering molecules in a variety of formats (i.e., space filling, skeletal, ball and stick) and also capable of providing quantitative information about the model/molecule (i.e. bond angles and lengths, energy, and interatomic distances). They also are unlimited in the size of the molecule that can be represented (as kits are). In the second part of the lab you will examine some of the physical and chemical properties, including water solubility, density, and reactivity with acids and bases, of some representative alkanes and alkyl halides. Definitions Conformation - A particular molecular structure that is arrived at through single bond rotation. Different conformations of a molecule are easily interconverted by single bond rotation(s). Conformer - A conformation of a molecule that corresponds to a potential energy minimum for the molecule. 1 Plane of Symmetry - Any object that consists of two halves that are mirror images of each other is said to possess a "plane of symmetry" or a "mirror plane". For example a human body, a fork, a baseball bat, and a basketball all possess mirror planes (neglecting small imperfections and markings on the objects). Examples of objects that do not have a plane of symmetry would be a human hand, a golf club, and a bowling ball. PROCEDURES FOR PART A. MOLECULAR MODELING Conformations of Ethane Construct a model of ethane, CH3CH3. Look straight down the C-C bond and rotate it until the C-H bonds in front exactly bisect the H-C-H angles of the back carbon. You now have a model of the staggered conformation of ethane This structure can be represented on paper in a number of ways, including wedge-dash formulas, Newman projections, and "sawhorse" or "andiron" formulas (see below). H H H H H H H H H H H H H H H H H H Newman projection Sawhorse or Andiron fomula Wedge/Dash formula Answer all questions directly on the report sheet as you do this experiment. 1. Does the staggered conformation of ethane possess any planes of symmetry? If so tell how many there are and describe their location(s) in the molecule. Now rotate one carbon atom on your model 60 with respect to the other carbon. You now have a model of the eclipsed conformation of ethane. 2. Draw eclipsed ethane (i.e., this model) using Newman, sawhorse, and wedge/dash formulas. 3. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. 4. In which of the two ethane conformations do the sigma electrons of the C-H bonds on one carbon come closer to the sigma electrons of the C-H bonds of the other carbon? 5. Taking your answer to #4 into consideration, which conformation would you expect to less stable, the eclipsed or the staggered? 6. What type of molecular motion is required to convert the eclipsed conformation to the staggered conformation? 7. Make a graph of potential energy versus the angle between one of the C-H bonds on the front carbon and one of the C-H bonds on the back carbon (the dihedral or torsion angle). Started with the eclipsed conformation (torsion angle = 0) and go through one full rotation (360). Indicate on the graph where each conformation would be found. 2 Conformations of Propane Remove a hydrogen from the ethane model and add a CH3 group in its place. You now have a model of propane. Rotate each of the C-C bonds so that both of these bonds are staggered. A wedge/dash formula of the model should look like the figure below. H H H H H H H H 8. Draw a Newman projection that represents this conformation of propane. Represent the extra methyl group simply by "CH3". (Note - the Newman projection should look the same regardless of which C-C bond you sight down. 9. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. Rotate one of the C-C bonds in this model so that it is now eclipsed. It should look like the figure below. H H H H H H H H 10. Would a single Newman projection be able to adequately represent this conformation of propane? 11. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. 12. As you did in #7, plot a graph of energy versus torsion angle for one of the C-C bonds in propane. (Assume the other C-C bond stays staggered.) 13. What would you expect the energy difference between staggered and eclipsed conformations of propane to be relative to the same energy difference in ethane? (Would it be larger, equal to, or smaller?) Explain. Now rotate the other C-C bond so that it is also eclipsed. 14. Draw a wedge/dash representation of this conformation of propane. 15. Draw a Newman projection of this conformation of propane. 16. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. 17. Of the three propane conformations examined (staggered-staggered, staggered-eclipsed, and eclipsed-eclipsed), which one is the least stable? Why? Conformations of Butane Remove a hydrogen from one of the end carbons of the propane model and add a CH3 3 group in its place. You now have a model of butane. Rotate each of the C-C bonds so that they are all staggered and the two end CH3 groups are opposite each other. A wedge/dash formula of the model should look like the figure below. This model H H H H H H H H H H represents the anti conformation of butane. For short we will refer to it as conformation A. 18. Draw a Newman projection that shows the conformation of the bond between the center two carbons (use "CH3" to represent each of the end carbons) 19. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. Now rotate the center C-C bond 60. This is conformation B. 20. Draw a Newman projection that shows the conformation of the bond between the center two carbons. 21. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. Rotate the center bond another 60. This model represents the gauche conformation of butane. For short we will refer to it as conformation C. 22. Draw a Newman projection that shows the conformation of the bond between the center two carbons. 23. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. Rotate the center bond another 60. This is conformation D. 24. Draw a Newman projection that shows the conformation of the bond between the center two carbons. 25. Are there any planes of symmetry present? If so tell how many there are and describe their location(s) in the molecule. 26. Which of the four conformations of butane (A, B, C, & D) are staggered? 27. Which of the four conformations of butane (A, B, C, & D) are eclipsed? 28. What is the order of stability (from least stable to most stable) of these four conformations? 29. As you did in #7 and #12, plot a graph of energy versus torsion angle of the central C-C bond in butane. Use the angle between end CH3 groups to define the torsion angle (i.e., D= 0, C= 60, etc). Make sure the graph goes through one full revolution of the C-C bond (goes from 0 to 360 degrees) and that it shows the relative energies of each conformation. 30. Which of the four conformations are properly referred to as "conformers"? (See the definitions at the start of this handout.) 4 Conformations of some other Compounds Now make a model of and then draw a Newman projection of the most stable conformer (around the C3-C4 bond) of each of the following compounds. 31. 2,5-dimethylhexane (1,2-diisopropylethane) 32. 2,2,5,5-tetramethylhexane (1,2-di-tert-butylethane) 33. In which of these two compounds is the energy difference between the anti conformer and gauche conformer the greatest? Explain why. Make a model of 2,3-dimethylbutane. 34. Consider the C2-C3 bond. There are two conformers of nearly equal stability for this molecule. Draw Newman projections of each. 35. Which of these two do you think is more stable? Explain why. PROCEDURES FOR PART B. PROPERTIES Obtain about 0.5 mL of each of the following compounds; hexane, octadecane, 1chlorobutane, 1,2-dichloroethane. (Make sure you label the test tubes you use as containers.) Observe each compound carefully and note descriptions of each in your notebook. Safety: Keep the tubes tightly corked and work in a fume hood if possible. Dispose of the tube contents when finished in the provided waste bottle in hood #1. Water Solubility and Density Test each compound in turn by adding about 0.2 mL of it to about 2 mL H2O in a small test tube. Cork the tube and shake for several minutes, allow the tube to sit undisturbed for a minute or so, and then note the result in your notebook. (If the compound does not dissolve and forms the top layer when mixed with water then the density should be noted as "d < 1". If it forms the bottom layer then d > 1.) Reactivity with NaOH(aq) Test each compound in turn by adding about 0.2 mL of it to about 2 mL concentrated NaOH(aq) in a small test tube. Cork the tube and shake for several minutes, allow the tube to sit undisturbed for a minute or so, and note the result. Look carefully for any sign of reaction such as a color change, gas evolution, or heat evolution. Reactivity with H2SO4(conc) Make sure you use dry, clean test tubes for this part. Test each compound in turn by adding 5-6 drops of concentrated sulfuric acid to around 0.1 mL of the compound. A significant color change (i.e. solution gets dark or turns black) and heat evolution would be indicative of a reaction. 5