壹、 - 國立彰化師範大學圖書館
advertisement

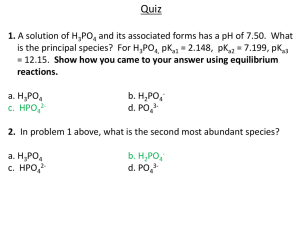
國立彰化師範大學九十三學年度博士班招生考試試題 系所:科學教育研究所 科目:基礎化學 組別:丙組 ☆☆請在答案紙上作答☆☆ 第 1 頁,共 3 頁 1. (12%) 0.1152 g of a compound containing carbon, hydrogen, nitrogen and oxygen are burned in excess oxygen. The gases produced are treated further to convert nitrogen-containing products into N2. The resulting mixture of CO2, H2O and N2 and excess O2 is passed through a CaCl2 drying tube, which gains 0.09912 g. The gas stream is bubbled through water where the CO2 forms H2CO3. Titration of this solution to the second endpoint with 0.3283 M NaOH requires 28.81 mL. The excess O2 is removed by reaction with copper metal and the N2 is collected in a 225.0 mL measuring bulb where it exerts a pressure of 65.12 mmHg at 25 °C. In a separate experiment the molar mass of this compound is found to be approximately 150 g.mol-1. a. Calculate the number of moles of (i). H2O (ii). CO2 (iii). N2 b. Determine the mass in the original compound of (i). C (ii). H (iii). N (iv). O c. Find the empirical formula of the compound. d. Find the molecular formula. 2. (12%) Phosphoric acid, H3PO4, ionizes according to the equations, H3PO4 (aq) H+ + H2PO4- (aq) K1 = 7.1 x 10-3 H2PO4- (aq) H+ + HPO42- (aq) K2 = 6.2 x 10-8 HPO42- (aq) H+ + PO43- (aq) K3 = 4.5 x 10-13 a. Write the equilibrium expression for the ionization of H3PO4 and find the pH of a 1.5 M solution of H3PO4. b. A student is asked to prepare a phosphate buffer with a pH of 7.00. Identify the species that should be used in this solution and calculate their ratio. c. Assume that 50.0 mL of the buffer solution in b. are available in which the more abundant buffer species has a concentration of 0.10 M. Determine the [H+] in this solution after 2.0 x 10-3 mol of NaOH are added. 3. (12%) Write net equations for each of the combinations of reactants below. a. Water is added to magnesium nitride. b. Excess carbon dioxide is bubbled through a solution of calcium hydroxide. c. Solutions of lead acetate and sulfuric acid are mixed. -1- 國立彰化師範大學九十三學年度博士班招生考試試題 系所:科學教育研究所 科目:基礎化學 組別:丙組 ☆☆請在答案紙上作答☆☆ 第 2 頁,共 3 頁 4. (16%) (A) Count the electrons and shows if the following compounds obey the rule of 18 (EAN rule). (i). [Pt(NH3)4]2+ (ii). (η5-C5H5)2Ni (iii). ReH92- (iv) TaMe5. (B)For which dn configurations would no Jahn-Teller splitting be expected for the tetrahedron case (ignore possible low-spin cases). (C) Give the bond order and number of unpaired electrons for (i) B2+ (ii) C2+ (iii) O225. (10%) The deoxy forms (without oxygen) of three oxygen carrying proteins are shown in the following diagram with (A) hemoglobin (B) hemerythrin (C) hemocyanin. Questions: (i) Describe the dioxygen binding modes and metal oxidation state after oxygen bound on each system. (ii) Suggest a method that can differentiate different binding mode of dioxygen. N N FeII N N (A) (B) (His)N HOOC H O N(His) FeII (His)N N(His) O N(His) OO O Glu Asp (His)N II Fe (C) (His)N (His)N CuI N(His) CuI N(His) N(His) N(His) HOOC 6.(16%) Complete the following reactions (A) cat. H+ O + OH HO (B) Predict the stereochemistry of the major product of the Diels-Alder reactions Me OAc CN Me (C) O CH3 Na, EtOH O liq. NH3 (D) Me O i-Pr MgCl cat. (Me2S)CuCl -2- 國立彰化師範大學九十三學年度博士班招生考試試題 系所:科學教育研究所 科目:基礎化學 組別:丙組 ☆☆請在答案紙上作答☆☆ 第 3 頁,共 3 頁 7.(10%) Given the following enthalpy and entropy data, calculate ΔGof of CH4 gas at 25 oC: ΔHof (25 oC ; kjoules/mole) So (25 oC, joules/oK-mole) C(graphite) 0 5.69 H2(g) 0 130.59 CH4(g) -74.85 186.20 8.(12%) Explain the following terms. (A). Hard-Soft Acid and Base Concept (B). Anti-Markovnikov addition (C). Stochiometry of chemical reactions -3-