Quiz 12
advertisement
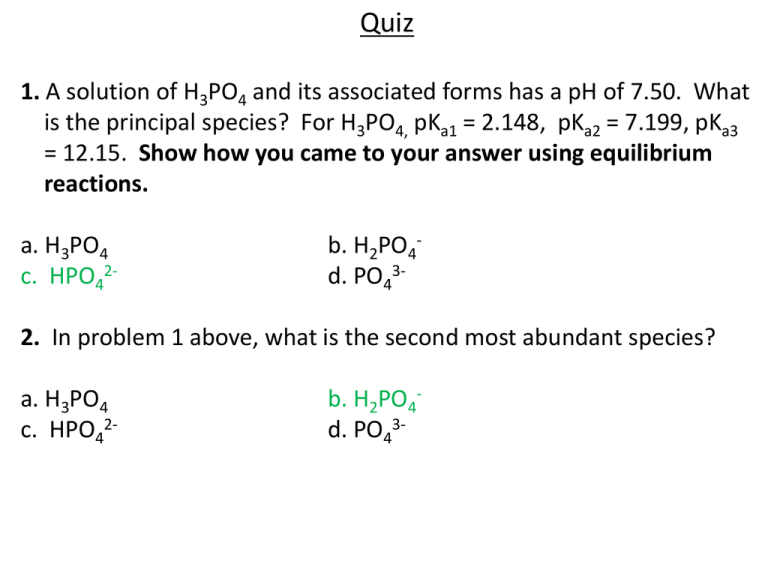
Quiz 1. A solution of H3PO4 and its associated forms has a pH of 7.50. What is the principal species? For H3PO4, pKa1 = 2.148, pKa2 = 7.199, pKa3 = 12.15. Show how you came to your answer using equilibrium reactions. a. H3PO4 c. HPO42- b. H2PO4d. PO43- 2. In problem 1 above, what is the second most abundant species? a. H3PO4 c. HPO42- b. H2PO4d. PO43- Quiz 3. How many mL of 0.250 M HCl need to be added to 20.0 mL of 0.350 M acetate to reach the equivalence point? Show your work. a. 14.0 mL c. 28.0 mL b. 20.0 mL d. none of the above 4. Consider the titration of a weak acid whose pKa is 7.7. What is the pH of the titration half way to the equivalence point? a. 7.7 c. 7.0 b. 6.3 d. more information is necessary