to view the document. - UROP - University of California, Irvine
advertisement

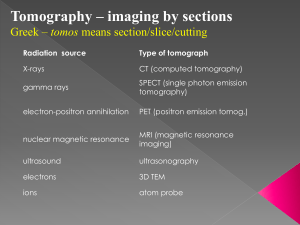
Abstract Air Force Portable Device for Retinal Imaging Military aircrew members, or anyone involved in a dangerous activity requiring very high visual performance, would benefit from a field-deployable screening device to detect lesions of the retina before they could cause permanent loss of visual sharpness or blindness. At present, there is no accepted diagnostic or screening protocol for ocular lesions and retinal cell loss associated with retinal laser injuries. Photographic fundus imaging is a standard procedure to evaluate ocular lesions; however, it can only provide a two-dimensional retinal surface image. The newly developed OCT technology can provide three-dimensional retinal images that will greatly facilitate the in-field diagnosis and treatment of ocular lesions caused by laser weapons or laser guided aiming devices. This technology uses a swept laser source for Fourier domain optical coherence tomography (FDOCT), with 1 m in tissue band probe light, high-contrast and three-dimensional for retinal imaging. This SS-OCT has a measurement speed of 20,000 A-lines/s, a depth axial resolution of 12 m in tissue and sensitivity of 99.3 db, and coherence length of 9.8 mm at 2 kHz sweep rate. A software-based algorithm of scattering optical coherence angiography (S-OCT) was used to visualize the 3-D retina of the human eye. Based on the swept source, an FDOCT system was developed that can achieve 12 m axial resolution in tissue. Imaging of a human retina was demonstrated with the FDOCT system, and the FDOCT resolution was improved by reducing the effects of the tails or side lobes using the wiener filter logarithm that was implemented in the S-OCT. Abdelhamid Jnane City College Of New York CUNY Air Force Portable Device for Retinal Imaging Mentors: Dr.zhongping Chen Dr. Qiang wang University of California, Irvine Beckman Laser Clinic Key words Optical Coherence Tomography (OCT) Fourier domain OCT Spectral domain OCT Swept Domain OCT Scattering OCT Interferometer Full-width at half maximum (FWHM) Gaussian Spectrum Wiener filter Introduction Optical coherence tomography is a non-invasive in vivo imaging technique, which has undergone rapid development since its invention in 1991 [1,2]. In 1991, optical coherence tomography (OCT) was reported for the first time [3]. Using a Michelson interferometer and a low coherence light source, OCT performs cross-sectional imaging of internal tissue microstructure by measuring the echo time delay of backscattered infrared light [4,5]. In an interferometer, light emitted from a source is divided by a beam splitter in two directions towards two mirrors, a reference mirror and a sample mirror. The back-reflected beams recombine at the beam splitter and are guided to a detector. When the reference arm is moved while the sample is fixed, an interference pattern is measured by the detector. Interference, however, will only be detected when the difference in optical distance traveled by the light in both arms is less than or equal to the coherence length of the light source [6,7]. The interferometer output is detected and demodulated to measure the echo time delay and magnitude of the backscattered light. By acquiring sequential transverse axial measurements and removing motion artifacts using crosscorrelation algorithms [8], the resulting data set is a two-dimensional array representing the optical backscattering in a cross-sectional plane of the tissue. This can be digitally processed and displayed as a gray-scale or false-color image [9]. There are two types of OCT. The first kind is called time domain OCT (TDOCT) [10], which is a single longitudinal scattering profile that translates the reference mirror. In this system, both the depth and transversal directions have to be mechanically scanned to register a two-dimensional cross-section map of the region of interest within the object [11,12,13]. Fourier domain OCT (FDOCT) is an alternative to TDOCT, which provides high speed and high sensitivity [14,15]. In FDOCT mechanical depth scans are not required. The reference mirror position is fixed and the spectrum of the light at the output of the Michelson interferometer is recorded by a spectrometer equipped with a high-speed multi-element detector. To provide the profile of the sample reflectance versus depth (optical A-scan), the Fourier transformation of the interference pattern is used. FDOCT detection can be performed in two ways: spectral domain OCT (spectral OCT), using a spectrometer with a multi channel analyzer [16,17], or swept source OCT, using a rapidly tunable laser source [18,19,20]. Spectral OCT and swept source OCT have been used for ophthalmic imaging in the anterior eye and retina and have demonstrated ultrahigh-resolution imaging and high speeds when compared to TDOCT [21]. The longitudinal resolution in OCT is proportional to the source spectral bandwidth, which governs the axial point-spread function. Therefore, the full-width at half maximum (FWHM) of the interference fringe envelope is used to define the axial resolution of an OCT system. Side lobes and tails in the interference fringe envelope are usually caused by the non-Gaussian spectral shape, due to several types of sources (e.g., semiconductor optical amplifier) of light. OCT resolution can be improved by reducing the effects of the tails or side lobes using some signalprocessing algorithm. The Wiener Filter logarithm was implemented in this study. [22]. FDOCT with a wavelength of 1.3 m is widely used in many ophthalmology applications because it provides higher penetration for a tissue than the one with an 830 nm wavelength. [23]. However, the 1.3 wavelength is not suitable for the investigation of retinal imaging because the water absorption of 1.3 m light is 38 times greater than that of 830-nm light [24]. Because water absorption has a local minimum at 1.06 m, wavelength with 1 m is the alternative solution for high penetration retinal OCT. The 1 m wavelength can easily access and travel in the vitreous body of the retina. Because SD-OCT requires a high-speed spectrometer, it is not suitable to assemble an SD-OCT with a 1 m probe. An SS-OCT scheme is the alternative for 1 m FDOCT because of its higher speed and higher tolerability in sampling the signal [25]. In this study, we describe the development of new FDOCT device combined with a 1 m swept source light to visualize 3-D retinal structure imaging. We also demonstrate a novel implementation of numerical spectral shaping using Wiener Filter algorithm to smooth the spectrum, and we conduct a preliminary testing of our system. Fig. 1. Schematic of the FDOCT system: Collimator; adjustable neutral density attenuator; D1 and D2; photodetectors. Spectrum envelope of the source 80 Axial PSf without spectral reshaping 200 Amplitude[arbitrary units] Intensity [Arbitrary units] 70 60 50 40 30 0 20 1020 1030 1040 1050 1060 1070 1080 1090 1100 1110 Wavelength [nm] 0 100 200 300 400 Miror displacement[mic.m] Fig. 2. (a). Non-gaussian Spectrum from the swept light source registered by the OCT spectrometer. (b). The curve represents the spectral uncorrected response for Coherence envelopes determined from glass slide Reflection. Method and System Hardware: The scheme of our FDOCT instrument (Fig.1) uses a 1 m swept laser source as a light source with booster and without booster (2 kHz repetition rate; Peak power of 0.7 to 4 mW; FWHM_ sweep bandwidth of 31 nm centered at 1078 nm without booster and 1061 nm with booster). The hardware specifications used in this OCT design were as follows: Collimator (OFR Inc., 1060 nm); Gold mirror (Thorlabs Inc., ½”); 2 circulators (Agiltron Inc., 1060 nm); 70/30 2x2 couplers (AC Photonics Inc. 1060 nm; 50/50 2x2 couplers (AC Photonics Inc. 1060 nm); X stage (Newport Inc.); Light source (Santec Inc., 1060 nm, 28 kHz); Aluminum case (Hammond Manufacturing, 10.03” x 9.63” x 3.84”). The two 50/50 fiber couplers were used to increase SNR of the system. The output light from the swept source was directed into reference and sample arms by a 2*2 coupler. An adjustable attenuator was used to attenuate the reference power for enhanced sensitivity in the reference arm. The imaging speed of the system is 20 k Alines/second and the power delivered to sample arm is 1.2 mw. The signal from the balanced detector was converted by a data acquisition board sampling at 10 MHz. The number of data points for each A-line data acquisition during the frequency scan was 1024. The axial resolution in free space was determined to be 17 m corresponding to the effective axial resolution of 12 m in tissue by measuring the point spread function with a partial reflector at a depth of 900 m. Numerical spectral reshaping: As shown in Fig. 2 light source has a non-gaussian shape caused by optical fiber components of the light source. To get a gaussian shape of the spectrum, we applied a numerical method by using a Wiener Filter. To design a Wiener Filter, it is essential to determine the envelope of the wavelength-dependent fringe contrast. First, we extracted the wavelength-dependent fringe contrast from the OCT spectral signals. The spectral interference signal can be expressed as follows: S j (k) = Se (k) cos j (k) (1) Where Se (k) is the contrast, cos j (k) is the fri j (k) is the phase of the j-th fringe. The ensemble average of S j(k)2 in j can be rewritten as follows: S j (k) 2j = Se (k)^ 2 cos^2 j (k)j (2) Since the squared cosine term can be reduced to a constant value of ½, the contrast envelope is rewritten as: –Se (k) =√ (2/N*Σ (j) Sj (k)^2 (3) Substitution of equation (3) in a Wiener Filter yields the desired spectral reshaping filter that will be implemented in our OCT software, as illustrated below: W (k) =(Se (k)/(Se (k) ^2 +Nc))*Gaussian (k) Where Nc is a constant depending on the SNR of the detection system and Gaussian (k) is a Gaussian window to reshape the spectrum to a Gaussian profile. For the Wiener Filter implementation for our OCT, we wrote a C++ program portion to reshape our spectral light source and obtain a smooth gaussian shape of the signal. For the spectral envelope calculation we used the following polynomial 8 degrees: Spectral Envelope = A + B1*j + B2*j^2 + B3*j^3 + B4*j^4 + B5*j^5 + B6*j^6 + B7*j^7+B8*j^8 Where A=23.84411; B1=0.95364; B2= -0.0062; B3=9.5098E 5; B4= -7.19185E 7; B5=2.49286E-9; B6= -4.32256E-12; B7=3.66599E-15;B8=1.21285E-18. We then express the Gaussian envelope and spectral-reshape as: Gaussian Envelope = 100*exp (-(j-425.0)* (j-425.0)/240.0/240.0) Spectral Reshape=Gaussian Envelope [(2.0*Spectral-Envelope +constant factor)]; Results and discussion Fig.3. A compact FDOCT portable device for retinal imaging 1.002 70 Specrtrum envelope of the source Axial PSF with spectral reshaping 60 Amplitude[arbitrary units] Intensity [arbitrary units] 1.000 0.998 0.996 0.994 0.992 50 40 30 20 10 0 0.990 1020 1030 1040 1050 1060 1070 1080 1090 1100 1110 Wavelength [nm] 0 100 200 300 400 Miror displacemen[mic.m] Fig. 4. (a) Gaussian Spectrum from the swept light source registered by the OCT spectrometer. (b) The curve represents the spectral corrected response for Coherence envelopes determined from glass slide Reflection. The rat animal model will be used for in vivo imaging of retinal damage to evaluate the proposed field deployable imaging device. A mode locked Titanium: Sapphire laser with 800 nm wavelength, 76 MHz repetition rate and 170-femtosecond pulse or Continuum MiniliteTM, Q-switched Nd: YAG laser of 532 nm wavelength, 1~15 Hz repetition rate and 3~5 nanosecond pulses will be used to create laser lesions in rat retina. We will use the ophthalmoscope threshold of 5.9mJ (Robert J. Thomas, Lasers in Surgery and Medicine 31:9-17(2002)) as standard lesion dosage. Three types of lesions will be created by exposing to 100%, 200% and 300% of the standard lesion dosage. For the exposure time of 0.25 s (controlled by an electric shutter) that will be used in our experiment, the laser power for the three different dosages will be 23.6 mw, 47.2 mw and 70.8 mw respectively. Three groups of animals (Group A created with 23.6 mw, 0.25 s; Group B created with 47.2 mw, 0.25 s; Group C created with 70.8 mw, 0.25 s) will be required. A total of five animals will be assigned to each group to evaluate the time response of the retinal lesion at each of the five time points: 0 h, 1 h, 5 h, 24 h and 48 h post injury. A total of 15 rats (three groups x five time points/group x one rat/time point) will be needed for this pilot study. To accommodate factors such as laser misalignment during the creation of retinal lesions, the same laser parameter will be used to create wounds on both eyes of the rat. The rats will be housed individually and the cage for each rat will be tagged to identify its group and lesion response time. The following procedures describe how the experiment will be performed on each rat. After general anesthesia, retinal lesions on both eyes will be created and then imaged at the time point assigned to the animal. When the post injury time point for imaging is greater than 1 hr, the animal will be recovered from anesthesia and then reanesthetized at the designated imaging time point. Although the retinal injury will cause no pain, the animal will be monitored for signs of squinting, scratching and rubbing of eye as well as the swelling of the eyes during the time intervals between retinal injury and imaging time point. For those animals with long response times (24 hr or 48 hr), the animals will be transported back to the animal housing facility. At the assigned response time, the animal will be transported back to the BLI-S306, reanethetized, and the photographic fundus images and the 3-D retinal optical coherence tomography images will be obtained for both eyes. Immediately after the imaging procedure, the animal will be euthanized without recovering from general anesthesia and the eyes will be harvested. For each anima,l the experimental end point is reached at this time. In the pilot experiments described above, a total of 30 photographic fundus images and 30 3-D OCT images will be documented for the 15 rats. Two groups of ophthalmologists will evaluate the images. The first group will evaluate only the photographic fundus image to make an evaluation of the retina injury. Their evaluations will be matched, compared and scored according to the histology evaluation of the specimen. The second group will evaluate both the photographic fundus images and 3-D OCT images to evaluate the retina injury. The evaluation will be matched, compared and scored to the histology evaluation. The improved effectiveness of the retinalscreening device that uses both fundus photography and OCT imaging can be derived as the ratio of the total scores of the second review group to the scores of the first review group. Conclusion We have developed a FDOCT device with a 1 m swept light source, which has the following specifications: Dimensions of 8” x 8” x 4”; sensitivity of 99.3 db; High speed (20,000 A-lines/s); High resolution (12 mm); coherence length of 9.8 mm at 2 kHz sweep rate. We have also shown that FDOCT resolution can be improved by reducing the effects of the tails or side lobes by implementing the Wiener Filter algorithm to get a gaussian shape of the spectral light source. The algorithms we have used take advantage of the convolution property of Fourier transformation. Therefore, extensive computation that can slow the speed of the OCT is not necessary References [1] W. Drexler, U. Morgner, F. X. Kartner, C. Pitris, S. A., Boppart, X. D. Li, E. P. Ippen, and J. G. Fujimoto, Opt. Lett. 24, 1221 (1999). [2] Drexler, W., Morgner, U., Ghanta, R.K., Ka¨ rtner, F.X., Schuman, J.S., Fujimoto, J.G., 2001. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 7, 502– 507.4126–4131. [3] Huang, D., Swanson, E.A., Lin, C.P., Schuman, J.S., Stinson, W.G., Chang, W., Hee, M.R., Flotte, T., Gregory, K., Pulia.to, C.A., 1991. Optical coherence tomography. Science 254, 1178–1181. [4] Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991). [5] R. Tripathi, N. Nassif, J. S. Nelson, B. H. Park, and J. F. de Boer, “Spectral shaping for non-Gaussian source spectra in optical coherence tomography,” Opt. Lett. 27, 406–408 _2002_. [6] B. L. Danielson and C. Y. Boisrobert, “Absolute optical ranging using low coherence interferometry,” Appl. Opt. 30, 2975–2979_1991_. [7] C. K. Hitzenberger, A. Baumgartner, and A. F. Fercher, “Dispersion induced multiple signal peak splitting in partial coherence interferometry,” Opt. Commun. 154, 179–185 _1998_. [8] M. Bashkansky, M. D. Duncan, and J. Reintjes, “Engineering and laboratory notes—Signal processing for improving field cross-correlation function in optical coherence tomography,” Appl. Opt. 37, 8137–8138 _1998 [9] Ko, T.H., Fujimoto, J.G., Duker, J.S., Paunescu, L.A., Drexler, W., Baumal, C.R., Pulia.to, C.A., Reichel, E., Rogers, A.H., Schuman, J.S., 2004b. Comparison of ultrahigh- and standardresolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology 111, 2033–2043. [10] Choma, M.A., Sarunic, M.V., Yang, C.H., Izatt, J.A., 2003. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 11, 2183– 2189. [11] Choma, M.A., Hsu, K., Izatt, J.A., 2005. Swept source optical coherence tomography using an all-.ber 1300-nm ring laser source. J. Biomed. Opt. 10, 44009. [12] Gurses-Ozden, R., Teng, C., Vessani, R., Zafar, S., Liebmann, J.M.Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 7, 502–507. [13] Fujimoto, J.G. et al. Optical biopsy and imaging using optical coherence tomography. Nature Med. 1, 970–972 (1995). [14] Boppart, S.A. et al. In vivo cellular optical coherence tomography imaging. Nature Med. 4, 861–865 (1998). [15] Tearney, G.J. et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science 276, 2037–2039 (1997). [16] Y. Yasuno, V. D. Madjarova, S. Makita, M. Akiba, A. Morosawa, C. Chong, T. Sakai, K. Chan, M. Itoh, and T. Yatagai, “Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments,” Opt. Exp. 13, 10,652–10,664 (2005). [17] M. Sarunic, B. Applegate, S. Asrani, and J. Izatt, “Quadrature Projection Full Range High Speed Fourier Domain Optical Coherence Tomography,” Invest. Ophthalmol. Vis. Sci., EAbstract 47, 2928 (2006). [18] M. V. Sarunic, B. E. Applegate, and J. A. Izatt, “Real-time quadrature projection complex conjugate resolved Fourier domain optical coherence tomography,” Opt. Lett. 31, 2426–2428 (2006). [19] Y. Wang, Z. C. J. Nelson, B. Reiser, R. Chuck, and R. Windeler, “Optimal wavelength for ultrahigh-resolution optical coherence tomography,” Opt. Exp. 11, 1411–1417 (2003) [20] A. Unterhuber, B. Povazay, B. Hermann, H. Sattmann, A. Chavez-Pirson, and W. Drexler, “In vivo retinal optical coherence tomography at 1040 nm — enhanced penetration into the choroid,” Opt. Exp. 13, 3252–3258 (2005), [21] J. S. Schuman, C. A. Puliafito, and J. G. Fujimoto, eds., Optical Coherence Tomography of Ocular Diseases, 2nd ed. (Slack Incorporated, 2004). [22] M. Wojtkowski, V. Srinivasan, J. G. Fujimoto, T. Ko, J. S. Schuman, A. Kowalczyk, and J. S. Duker, “Threedimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography,” Ophthalmology 112, 1734–1746 (2005). [23] Y. Yasuno, V. D. Madjarova, S. Makita, M. Akiba, A. Morosawa, C. Chong, T. Sakai, K. Chan, M. Itoh, and T. Yatagai, “Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments,” Opt. Exp. 13, 10,652–10,664 (2005) [24] M. Sarunic, B. Applegate, S. Asrani, and J. Izatt, “Quadrature Projection Full Range High Speed Fourier Domain Optical Coherence Tomography,” Invest. Ophthalmol. Vis. Sci., EAbstract 47, 2928 (2006). [25] M. V. Sarunic, B. E. Applegate, and J. A. Izatt, “Real-time quadrature projection complex conjugate resolved Fourier domain optical coherence tomography,” Opt. Lett. 31, 2426–2428 (2006). Acknowledgements Dr. Zhongping Chen Dr. Qiang Wang University of California, Irvine Beckman Laser Institute IM-SURE Program Dr. Said Shokair NSF