Physical properties of transition metals and their compounds
advertisement

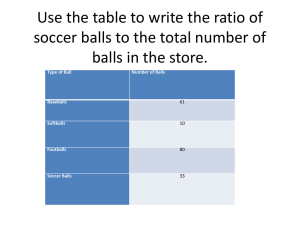
% composition and molecular formulae Simple ideas Imagine you have a boy and a girl in a class. The % of boys in the class = number of boys / total in class The % of boys in the class = 1/2 x 100 = 50% In this example we have 6 green and 3 orange balls % of green balls = number of green balls / total number of balls x 100 % of green balls = 6/9 x 100 = 66.7% % of orange balls = number of orange balls / total number of balls x 100 % of orange balls = 3/9 x 100 = 33.3% CHECK that the total % = 66.7% + 33.3% = 100% In this example we have 8 blue, 12 yellowish green and 5 orange balls. Total number of balls = 8 + 12+ 5 = 25 % of blue balls = 8 / 25 x 100 = 32% % of yellowish green balls = 12/25 x 100 = 48% % of orange balls = 5 / 25 x 100 = 20% CHECK that the total % = 32% + 48% + 20% = 100% SUMMARY We can find the % composition (by mass) of a substance if we know the chemical formula of the substance the mass of the substance the amount and mass of each of the elements making up the substance. EXAMPLE 1 Find the % composition of the elements present in water H2O. The chemical formula of the substance =H2O. The mass of the substance = 2 x 1 + 16 = 18g The mass of oxygen = 16g - hence % of oxygen in water = 16/18 x 100 = 88.9% The mass of hydrogen = 2g - hence % of hydrogen in water = 2/18 x 100 = 11.1 % If we put this in the form of a table: Elements Atomic Mass number of mass of element % composition of the element present of element (g) atoms present in compound (g) present in the compound Hydrogen 1 2 2x1=2 2 / 16 x 100 = 11.1% Oxygen 16 1 1 x 16 = 16 16 / 16 x 100 = 88.9% TOTAL MASS is 16 + 2 = 18g TOTAL % is 11.1+ 88.9 = 100% EXAMPLE 2 : Find the % composition of the elements present in ethanol C2H5OH. If we put this in the form of a table: Elements Atomic Mass number of mass of element in % composition of the present of element (g) atoms compound (g) element present in the present compound Hydrogen 1 6 6x1=6 6 / 46 x 100 =13.0 % Oxygen 16 1 1 x 16 = 16 16 / 46 x 100 =34.8 % Carbon 12 2 2 x 12 = 24 24 / 46 x 100 = 52.2% TOTAL MASS is % TOTAL = 6+16 + 24 = 46g 13+ 34.8+ 52.2 = 100% Exercise on % composition ChR3h1.htm Percentage Formulae (1) Work through the following exercise checking how you get on. 1.Find the % composition of the elements present in methanol CH3OH. (Atomic masses C = 12, H = 1, O = 16). Compound Atomic No. of atoms Mass of Total mass of % composition of each mass of of element element in compound element in the compound element present compound C = 12 C= C= CH3OH H=1 0 = 16 2.Find the % composition of the elements present in ethanoic acid CH3 COOH. (Atomic masses C = 12, H = 1, O = 16). Compound Atomic No. of atoms Mass of Total mass of % composition of each mass of of element element in compound element in the compound element present compound C = 12 C= C= C= CH3 COOH H=1 H= 0 = 16 O= 3. % composition of various fertilisers Which of the fertilisers below contains the greatest percentage of nitrogen (Atomic masses C = 12, H = 1, O = 16, N = 14). Fertiliser Formula Atomic mass of element Ammonia NH3 N = 14 Urea Ammonium Nitrate No. of atoms of nitrogen present Mass of Total mass nitrogen in of compound compound % composition of nitrogen in the compound N= % N= % N= % CO(NH2)2 NH4NO3 Hence the fertiliser with the most nitrogen in it is ......................................................................