= 5.90 x 1022 Au atoms = 19.3 g/cm3
advertisement

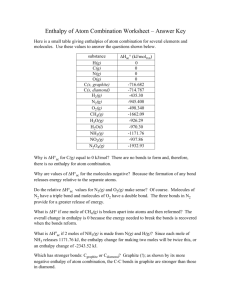
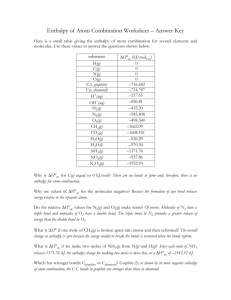
1. Ethylene glycol, the major ingredient in antifreezes at -11.5˚C. What is the freezing point in (a) K, (b) ˚F. (10%) K = ˚C + 273.15 ˚F = 5 ˚C + 32˚ 9 -11.5˚C = 261.65 K = 11.3 ˚F 2. Draw a modern periodic table including the atomic symbols for the elements with atomic numbers 1-20, and all the alkali metals, alkali earth metals, halogens, nobel gases, and 3 d transition metals. (20%) 3. A cube of gold that is 1.00 cm on a side has a mass of 19.3 g. A single gold atom has a mass of 197.0 amu. (a) How many gols atoms are in the cube ? (5%) (b) What is the density of gold? (5%) (c) From the information given, estimate the diameter in Å of a single gold atom. (5%) (a) 19.3 g 22 = 5.90 x 10 Au atoms -24 197.0 amu x1.66 x 10 g (b) D = (c) 3 M 19.3 g 3 = = 19.3 g/cm 3 V 1 cm 5.9 x 10 22 = 3.89 x 107 Au atoms, 1 cm = 2.57 x 10-8 cm = 2.57 Å 7 3.89 x 10 4. Determine which of the following compounds are soluble in water: (i) PbNO3 (ii) CaCl2 (iii) BaSO4 (iv) CuCO3 (v) Ag(C2H3O2) (10%) (i) PbNO3 (ii) CaCl2 (v) Ag(C2H3O2) 5. Write the balanced equations for the following reactions (20%) (i) gold dissolved in aqua regia Au(s) + NO3-(aq) + 4H+(aq) + 4Cl-(aq) → AuCl4-(aq) + 2H2O(l) + NO(g) (ii) complete combustion of propane C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) (iii) the action Alka-Seltzer with stomach acid NaHCO3(aq) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g) (iv) Nickel metal with hydrochloric acid. Ni(s) + 2HCl(aq) → NiCl2(aq) + H2(g) 6. (a) What is the first law of thermodynamics? (b) Why is the enthalpy change equal to the heat gained by the system at constant pressure when only P-V work is involved? (10%) (a) Energy is conserved. Any energy that is lost by the system must be gained by the surroundings, and vice versa. ∆E = q + w E : internal energy q: when heat is transferred to the system from the surrounding, q has a positive value w : when work is done in the system by the surroundings, w has a positive value (b) When a change occurs at constant pressure, the change in enthalpy, ∆H. Is given by the following relationship: ∆H = ∆ (E+ PV) = ∆E + P∆V (The work involved in the expansion or compression of gases is w = -P∆V) ∴ ∆H = ∆ (E+ PV) = (qP + w) – w = qP 7. (a) What is Hess’s Law? (5%) (b) What is the enthalpy of formation?(5%) (c) Calculate ∆H for the reaction C2H4(g) + 6F2(g) → 2CF4(g) + 4 HF(g) (5%) (a) If a reaction is carried out in a series of steps, ∆H for the overall reaction will equal the sum of the enthalpy changes for the individual steps. (b) Enthalpy of formation is the enthalpy change that occurs when a compound is formed from its component elements. (c) ∆H = (1) x 2 + (2) x 2 + (3) x (-1) = (-537 kJ) x 2 + (-680 kJ) x 2 + (-52.3 kJ) x (-1) = -2381.7 kJ 8. (a) Calculate the ionization energy of the ground-state hydrogen atom (in kJ/mol) (5%) (b) Calculate the wavelengths (in nm) of the first three lines in the Balmer series. (10%) 1 1 (a) ∆E = (-hcRH) ( - 2 ) 2 nf ni = (-2.18 x 10-18 J) ( 1 1 ) ∞ 12 3 = 2.18 x 10-18 J = 1.31 x 10 kJ/mol (b) Balmer series : n1 = 2, n2 = 3, 4, 5 …… (i) ni = 3, nf = 2 (6.63 x 10 -34 J - s) (3.00 x 10 8 m ) hc s = 6.56 x 10-7 m = 656 nm = λ= 1 1 ∆E - (-2.18 x 10 -18 J) ( 2 - 2 ) 2 3 (ii) ni = 4, nf = 2 λ= hc = 4.86 x 10-7 m = 486 nm ∆E (iii) ni = 5, nf = 2 λ= hc = 4.34 x 10-7 m = 434 nm ∆E 9. Use the de Broglie relationship to determine the wavelengths (in m) if the following objects: (a) an 85-kg person skiing at 50 km/hr, (b) a 10.0-g bullet fired at 250 m/s, (c) a lithium atom moving at 2.5 x 105 m/s (10%) (a) λ= h 6.63 x 10 -34 J - s -37 = = 5.6 x 10 m m mv 85 kg x 13.89 s (b) λ= h 6.63 x 10 -34 J - s -34 = = 2.65 x 10 m -3 mv 10 x 10 kg x 250 m s (c) λ= h 6.63 x 10 -34 J - s -13 = = 2.3 x 10 m - 27 5 m mv (6.941 x 1.66 x 10 kg) x 2.5 x 10 s 10. What scientific achievements were awarded in this year’s (2005) Nobel Prizes for Chemistry and Physiology or Medicine. (10%) (i) Chemistry : for the development of the metathesis method in organic synthesis. (ii) Physiology or Medicine : for the discovery of the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer disease.