Physical Chemistry Final Exam - January 2007
advertisement

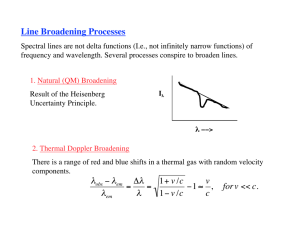
Physical Chemistry Final January 2007 * Show details of your calculation and analysis (you MUST specify the units clearly). 1. (10%) Explain the reasons for the broadening of the widths of spectral lines: (a) Doppler broadening, and (b) lifetime broadening (uncertainty broadening and natural linewidth). (c) How can we improve the resolution of the spectra ? 2. (10%) Calculate the frequency of the J = 5 4 transition in the pure rotational spectrum of 14N16O. The equilibrium bond length is 115 pm. 3. (10%) Calculate the percentage difference in the fundamental vibration wavenumber of 23Na35Cl and 23Na37Cl on the assumption that their force constants are the same. 4. (10%) Why don’t N2 and O2, the most abundant molecules, contribute to global warming ? 5. (10%) Calculate the relative numbers of Cl2 molecules ( v = 559.7 cm-1) in the ground and first excited vibrational states at (a) 298 K, (b) 3000 K. 6. (10%) How do the band heads in P and R branches arise? Could the Q branch show a head? 7. (10%) Explain the origin of the term symbol 3Σg- for the ground state of dioxygen. 8. (10%) A swimmer enters a gloomier world (in one sense) on diving to greater depths. Given that the mean molar absorption coefficient of sea water in the visible region is 6.2 10-5 dm3 mol-1 cm-1, calculate the depth at which a diver will experience (a) half the surface intensity of light, (b) one tenth the surface intensity. 9. (10%) Compare the relative stability of butadiene and cyclobutadiene using Hückel theory. 10. (5%) The molar absorption coefficient of a solute at 540 nm is 286 dm3 mol-1 cm-1. When light of that wavelength passes through a 6.5 mm cell containing a solution of the solute, 46.5 per cent of the light is absorbed. What is the concentration of the solution? 11. (5%) Which of the following molecules may show infrared absorption spectra: (a) H2, (b) HCl, (c) CO2, (d) H2O? Constants: c = 2.998×108 m s-1 u = 1.66054×10-27 kg h = 6.626×10-34 J s k = 1.38065×10-23 J K-1 NA = 6.02214×1023 mol-1 1