Zofran%20ODT%20from%20MicroMedex[1].
advertisement
![Zofran%20ODT%20from%20MicroMedex[1].](http://s3.studylib.net/store/data/007343189_1-0eed6d95a0696b540be9265cd1cf4ab1-768x994.png)
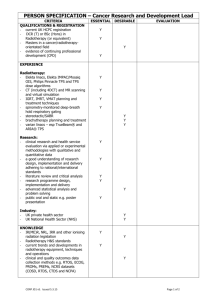
Zofran ODT from MicroMedex Adult Dosing (orally disintegrating tablets) ondansetron tablets, oral disintegrating tablets, and solution are bioequivalent; the dosages are interchangable for tablets, orally disintegrating tablets, and oral solution [1] Chemotherapy-induced nausea and vomiting, Highly emetogenic chemotherapy; Prophylaxis: 24 mg dissolved ORALLY on tongue 30 min prior to the start of a single-day chemotherapy [2][1] Chemotherapy-induced nausea and vomiting, Moderately emetogenic chemotherapy; Prophylaxis: 8 mg dissolved ORALLY on tongue 30 min prior to chemotherapy and repeated in 8 hr, then 8 mg every 12 hr for 1 to 2 days post chemotherapy [2][1] Postoperative nausea and vomiting; Prophylaxis: 16 mg dissolved ORALLY on the tongue 1 hr before anesthesia induction [1][2] Radiation-induced nausea and vomiting; Prophylaxis: (daily fractionated radiotherapy to abdomen) 8 mg dissolved ORALLY on tongue 1 to 2 hr prior to radiotherapy and every 8 hr after first dose for each day radiotherapy is given [2][1] Radiation-induced nausea and vomiting; Prophylaxis: (single high-dose fraction radiotherapy to abdomen) 8 mg dissolved ORALLY on tongue 1 to 2 hr prior to radiotherapy and every 8 hr after first dose for 1 to 2 days after radiotherapy completion [2][1] Radiation-induced nausea and vomiting; Prophylaxis: (total body irradiation radiotherapy) 8 mg dissolved ORALLY on tongue 3 times daily given 1 to 2 hr prior to each fraction of radiotherapy administered each day [2][1] Dose Adjustments renal impairment: dose adjustments are not necessary; there is no experience beyond the first day of administration [2][1] hepatic impairment, severe: (Child-Pugh score 10 or greater); do not exceed 8 mg/day [2][1] geriatric patients, 65 years of age or older: dose adjustments are not necessary [2][1] Contraindications concomitant use of apomorphine (Apokyn) hypersensitivity to ondansetron [2][1] Precautions chemotherapy-induced nausea and vomiting; symptoms of progressive ileus and/or gastric distension may be masked [2][1] congenital long QT syndrome; avoid use [5] cross-sensitivity among selective 5-HT3 receptor antagonists has occurred [2][1] do not use instead of nasogastric suction; does not stimulate gastric or intestinal peristalsis [2][1] ECG changes, including QT prolongation and potentially fatal Torsade de Pointes, have been reported; monitoring recommended in patients with electrolyte abnormalities (eg, hypokalemia, hypomagnesemia), bradyarrhythmias, congestive heart failure, and those taking concomitant medications that prolong the QT interval [5] following abdominal surgery; symptoms of progressive ileus and/or gastric distension may be masked [2][1] hepatic impairment, severe; dosage adjustment recommended [2] phenylketonurics; ondansetron orally disintegrating tablets (Zofran ODT(R)) contain phenylalanine [1] Pregnancy Category B Common Adverse Effects Gastrointestinal: Constipation, Diarrhea Hepatic: Increased liver enzymes Neurologic: Headache Other: Fatigue, Malaise Serious Adverse Effects Cardiovascular: Electrocardiogram abnormal, Prolonged QT interval, Torsades de pointes Immunologic: Anaphylaxis Respiratory: Bronchospasm Administration (oral disintegrating tablets) with dry hands, peel back foil backing and gently remove tablet; do not push oral disintegrating tablet through foil backing; administer immediately on tongue and oral disintegrating tablet dissolves in sec; swallow with saliva; liquid not required [1] (oral soluble film) with dry hands, fold the pouch and tear along the edge of the dotted line; remove film and place immediately on tongue; film dissolves in 4 to 20 sec; swallow with saliva; liquid not required; when more than one film is needed to make the total dose (16-mg or 24-mg dose), allow each oral soluble film to completely dissolve prior to taking the next film [2]
![[Physician Letterhead] [Select Today`s Date] . [Name of Health](http://s3.studylib.net/store/data/006995683_1-fc7d457c4956a00b3a5595efa89b67b0-300x300.png)