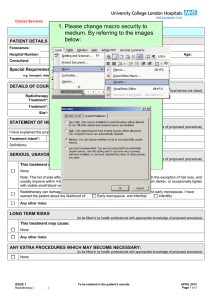
Person Specification - CPUK Template
advertisement
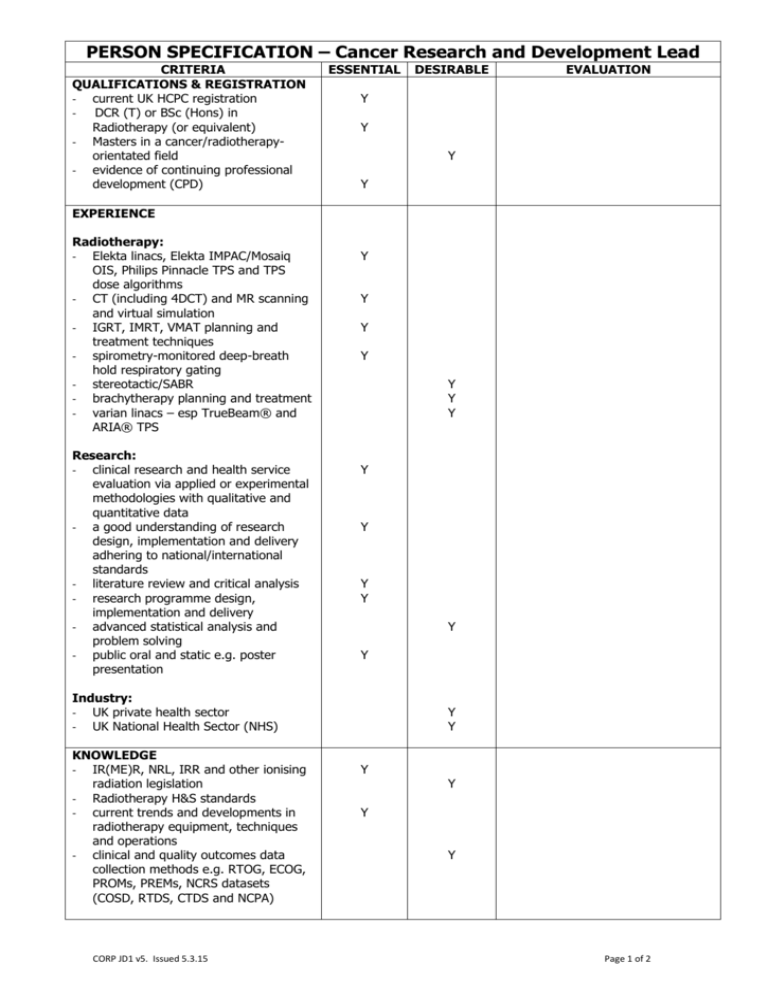
PERSON SPECIFICATION – Cancer Research and Development Lead CRITERIA QUALIFICATIONS & REGISTRATION - current UK HCPC registration - DCR (T) or BSc (Hons) in Radiotherapy (or equivalent) - Masters in a cancer/radiotherapyorientated field - evidence of continuing professional development (CPD) ESSENTIAL DESIRABLE EVALUATION Y Y Y Y EXPERIENCE Radiotherapy: - Elekta linacs, Elekta IMPAC/Mosaiq OIS, Philips Pinnacle TPS and TPS dose algorithms - CT (including 4DCT) and MR scanning and virtual simulation - IGRT, IMRT, VMAT planning and treatment techniques - spirometry-monitored deep-breath hold respiratory gating - stereotactic/SABR - brachytherapy planning and treatment - varian linacs – esp TrueBeam® and ARIA® TPS Research: - clinical research and health service evaluation via applied or experimental methodologies with qualitative and quantitative data - a good understanding of research design, implementation and delivery adhering to national/international standards - literature review and critical analysis - research programme design, implementation and delivery - advanced statistical analysis and problem solving - public oral and static e.g. poster presentation Y Y Y Y Y Y Y Y Y Y Y Y Y Industry: - UK private health sector - UK National Health Sector (NHS) KNOWLEDGE - IR(ME)R, NRL, IRR and other ionising radiation legislation - Radiotherapy H&S standards - current trends and developments in radiotherapy equipment, techniques and operations - clinical and quality outcomes data collection methods e.g. RTOG, ECOG, PROMs, PREMs, NCRS datasets (COSD, RTDS, CTDS and NCPA) CORP JD1 v5. Issued 5.3.15 Y Y Y Y Y Y Page 1 of 2 SKILLS - advanced problem-solving - autonomous and team-working - able to train clinical colleagues (doctors, radiographers, dosimetrists, technologists, physicists, etc.) - able to present and explain clinical and technical aspects of radiotherapy planning to non-clinical/non-technical staff (eg BDMs, visitors, patients) - able to engage with and maintain formal academic third-party collaborative research and peer-review Y Y Y Y Y Y GENERAL - liaise with the clinical, dosimetry and radiographer teams regarding existing data - maintain clinical benefits assessment projects via: collection of baseline data, collation and analysis supporting current projects e.g. DIBH and IGIMRT - provide suggestions on, and assist in, developing further research suitable for showcasing our continued quest for advancement in clinical excellence - maintain clinical skillset and experience as it is expected that a proportion of the hours will be attributed to clinical service - maintain own Continuing Professional Development (CPD) in accordance with CPUK and professional standards Y Y Y Y Y Person Specification approved by: Manager: Date: CORP JD1 v5. Issued 5.3.15 Page 2 of 2