The origin of depletion forces: entropy vs. enthalpy
advertisement

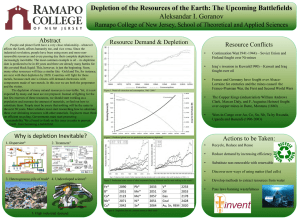
The origin of depletion forces: entropy vs. enthalpy Daniel Harries Institute of Chemistry and The Fritz Haber Research Center The Hebrew University, Jerusalem 91904, Israel E-mail: daniel.harries@mail.huji.ac.il Solutes preferentially excluded from macromolecules can drive depletion attractions in important biological association processes. The established Asakura-Oosawa theory relates depletion forces to the excluded volume reduction and the ensuing entropy gain upon macromolecular compaction. Accordingly, cosolute-induced protein stabilization is often described in terms of entropically driven “crowding”. In agreement, many experiments of protein folding and other macromolecular processes suggest that depletion forces are predominantly entropic for some cosolutes, such as polyethylene glycol polymers. However, for other cosolutes, such as polyol osmolytes, the effect is enthalpically dominated, while the entropic change can even be unfavorable. Using the Kirkwood-Buff theory of solutions we demonstrate that depletion forces can be quantified using the effective interaction between cosolute and macromolecule. Specifically, by incorporating interactions beyond hard-core, the depletion force attains considerable enthalpic contributions. This analytic theory, supplemented by Monte-Carlo simulations, traces the origins of enthalpically dominated depletion forces to "soft" cosolute-macromolecule repulsions. Moreover, these depletion forces can be entropically disfavoured if the effective cosolute-macromolecule interaction consistes of an entropic attractive component and an enthalpic repulsive component. Finally, changes in excluded volume upon compaction are found in the general case do not directly correspond to partial molar volumes. These findings suggest a modified view of the role of excluded cosolutes on macromolecular stabilization.