Diffusion and Osmosis Lab
advertisement

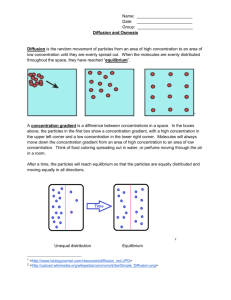
Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how different concentration gradients affect the movement of substances through semi-permeable membranes. 3. To understand how the processes of diffusion and osmosis through semipermeable membranes contribute to homeostasis in single-celled, aquatic organisms I. BACKGROUND MATERIAL All cells are separated from their external environment by a lipid membrane. Cell membranes have the challenging task of regulating the molecular traffic in and out of the cell. As a result of their supra-molecular structure (many molecules ordered into a higher level or organization), these membranes exhibit the property of selective permeability. Selective permeability refers to the ability of certain molecules to pass through the membrane while other molecules are blocked by the membrane or must be actively transported through the membrane. In this lab, we will examine the property of selective permeability utilizing dialysis tubing, water, and a set of solutions designed to reproduce situations unicellular organisms are faced with. TERMS Diffusion: The movement of a substance from a region of high concentration to a region of low concentration. Solute: A substance that is dissolved in a solution. Solution: A homogeneous, liquid mixture of two or more substances. The relative solute concentrations between two solutions are compared as: Hypotonic solution: A solution with a lower solute concentration than another solution. Hypertonic solution: A solution with a higher solute concentration than another solution. Isotonic solutions: Solutions of equal solute concentration. Osmosis: The diffusion of water across a semi-permeable membrane. Semi-permeable Membrane: A membrane that allows some molecules, but not others, to pass through it. Homeostasis: The inherent tendency in an organism to maintain physiological balance . 1 Diffusion and Osmosis II. FORMING HYPOTHESES 1. Make observations about the natural world. 2. Ask questions about those observations. 3. Formulate a reasonable testable hypothesis to explain observations. 4. Create, execute, and replicate experiments testing the hypothesis by generating data. 5. Analyze data in comparison to hypothesis and draw inferences. This stimulates further inquiry. The cycle begins anew. Observation: All living cells are surrounded by lipid membranes. These lipid membranes are semi-permeable, permeable to some molecules, and not others, which are determined by molecular size and charge. This semi-permeability controls diffusion and osmosis. Dialysis tubing can be used as a model for cellular membrane, and it sorts by size. Question: How do semi-permeable membranes and the processes of diffusion and osmosis contribute to homeostasis in single-celled, aquatic organisms? How does the molecular size of a solute affect these processes? How does the concentration of solute affect the rate of diffusion and osmosis? What happens if one solute can pass through the membrane, but another can’t? Hypotheses: 1. Because of the size of the pores in the dialysis tubing, starch should be too large to pass through the semi-permeable membrane, but glucose and iodine should be small enough to pass through the membrane. 2. The rate of osmosis increases with the concentration gradient across the membrane. Experiment: We will utilize solutions of starch, glucose, sucrose, and iodine to test these hypotheses using dialysis tubing as a model of the membrane of cells. Below, we lay out basic techniques that you can use to structure experimental protocols. 2 Results: Record your results in self-explanatory tables and graphs. Do your results differ from/ agree with your classmates? What inferences can you make from your own results or from compiled results? Diffusion and Osmosis III METHODS Part 1: Because of the size of the pores in the dialysis tubing, starch should be too large to pass through the semi-permeable membrane, but glucose and iodine should be small enough to pass through the membrane. Before we start, we should mention a little chemistry. When glucose or starch is dissolved in water they are colorless (or milky white). Starch and iodine react when in solution and turn blue in color. Additionally, we can test for the presence of glucose in solution with a urinalysis test strip. These will help you to determine what solutes are passing through the semipermeable membrane. You will set up an experiment where you fill a dialysis tube with starch and glucose, and submerge it in a beaker full of water and iodine solution. Think about this experimental design, and make predictions about what results you might expect to see from your experiment, based on the hypothesis you are testing. Be sure to record your observations in Table 2 Experimental procedure: Table 1. Part I materials Materials needed: Beaker Potassium iodide solution (IKI) Glucose Test Strips Dialysis tubing Glucose/starch solution Clamps Amount needed per group: 1 2 or 3 drops 4 1 – 6 inch strip 10-15ml 2 3 1. Fill a beaker about 2/3 full of water and add a few drops of Iodinepotassium iodide (IKI) solution. 2. Use one glucose test strip to test for presence of glucose in the beaker. Dip the strip into the solution, remove immediately, and compare the color with the scale on the bottle at 30 seconds. 3. Take one piece of soaked dialysis tubing, fold the end over on itself about 1”, and then close with a clamp 4. When you are ready to fill the dialysis tube, rub the open end of the tube between your fingers to open it up. Use a pipet to fill the tube with 10-15ml of the glucose/starch solution. 5. Use one glucose test strip to test for the presence of glucose in the solution you have just put into the dialysis tube. 6. Clamp the open end of the dialysis tube, and then place it into the beaker of water and iodine for 15-30 minutes. After 15-30 minutes, record the color of the liquids in the beaker, and the tube, then test the solution in the beaker with glucose test strips. Table 2. Data Table for Part I: Beaker Contents Color Start After 30 min. Color Records Beaker Contents Glucose Test Strip of Liquid Contents Solution In Dialysis Tube Dialysis Tube Glucose Test Color Strip Don’t test Thought questions: 1. If the dialysis tubing represents a single-celled aquatic organism, how would your results affect how a single-celled aquatic organism maintains homeostasis? 2. What do the contents of the beaker represent, i.e. the solution outside of the dialysis tubing? 3. Which substance(s) was/were able to pass through the dialysis tubing? Refer to experimental evidence to support your answer. Were your predictions correct? Did you support or reject the hypothesis you were testing? 4. If, a molecule is able to pass out of the dialysis tubing, do you think it would be able to pass the other way? (In other words, is the dialysis tubing a 4 one-way path?) Describe how you could alter the above experiment to determine if the dialysis tubing is semi-permeable one-way or two-ways. Part 2: The rate of osmosis increases with the concentration gradient across the membrane. Background: You will be working with different concentrations of starch and looking at how they affect diffusion across a semi-permeable membrane, the dialysis tubing. This part of the experiment is not completely written out and relies on you to establish the protocol. Don’t worry; we will give you some hints to get you started. Sucrose (common table sugar) is a molecule composed of one glucose molecule, and one fructose molecule. If a glucose molecule were able to pass through a membrane, would it be safe to assume the sucrose would also? What about the other way around? First, you need to think about how you will be able to quantify the amount of diffusion that occurs between the different concentrations. Table 3 lists the materials that are available for your experiment. After discussing possible protocols with your group, and your TA, outline the experiment you will conduct to test your hypothesis about how the concentration gradient affects the movement of water through a semipermeable membrane. (Hint: You will also want to set up a table to record your results and graph your final results in your notebook.) Based on lecture and results from your first experiment, formulate an explicit prediction(s) about what you expect to have happen in this experiment. Write it down here: 5 Table 3. Part 2 materials available Materials: 0.2 M sucrose solution 0.4 M sucrose solution 0.8 M sucrose solution 1.0 M sucrose solution Dialysis tubing Electronic scale 10 ml pipets 2.0 ml pipets Equipment stands Amount per group: 15ml 15ml 15ml 15ml 2 feet 1 1 4 Part 3: Real cell membranes are lipid bilayers that we believe will block the movement of charged ions like sodium and calcium. Water can pass through cell membranes. We will test this hypothesis using table salt (NaCl) and water. The cellular membranes of red onions are semi-permeable, permitting hydrophobic (uncharged) but not hydrophilic (charged) molecules or ions to pass. Sodium ions cannot pass through, but water can. Table 4. Part 3 materials Materials needed: Red Onion Slice Razor Blade Slide Cover Slip Microscope Amount needed per group: 1-2 cm2 1 1 1 1 1. Cut a small square out of a layer of the red onion (1-2 cm2). 2. Slice off/peel the thin red layer on the outside of the square using a razor. The thinner the layer, the better, so take your time. 3. Place the sample on a slide and try to keep it from folding over. Place a cover slip over the sample. 6 4. Place the slide on the microscope and focus to the layer of cells that are filled with red pigment. Try to find an area that you can clearly see individual cells. 5. Draw the cellular structure of these cells in your notes (note your magnification). If you look closely, you can see cell nuclei too. 6. Without removing the slide from the scope, drip a drop or two of 15%NaCl onto the edge of the cover slip. It should be pulled under the coverslip by capillary action, and cover your onion layer. 7. Continue to watch the cells for the next few minutes. You may want to move your slide around to see other areas for the layer since they may not all react the same way. Record your observations. Thought Questions: 1. Was the cell hypo-, hyper-, or isotonic relative to the NaCl solution? Refer to experimental evidence to support your answer. 2. What do you think would happen to a single-celled freshwater organism if it were suddenly thrown in the ocean? Refer to experimental evidence to support your answer. 3. Early life evolved in the sea before moving onto land. What are the challenges faced by a single-celled aquatic organism that tries to live on land? Please explain with reference to the following terms: osmosis, diffusion, solute concentration, and semi-permeable membrane. 7