Lab Experiment #5- Molecular Models and Polarity
advertisement

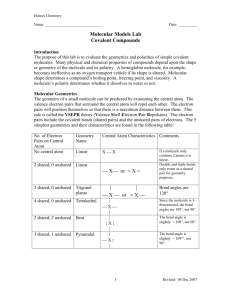
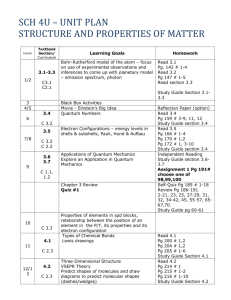
Jack M. Barrack Hebrew Academy 2013 – 2014, Tri 2 Honors Chemistry Dr. Darin Katz Lab Experiment #5- Molecular Models and Polarity The objectives of this lab experiment are to: to construct models of covalently-bonded molecules to examine the polarity of bonds to use models to determine molecular polarity General Instructions1. Please read the attached lab sheets so that you are familiar with the procedure(s) and what types of data you will collect. 2. Please answer the pre-lab questions on the next page after reading the lab. Your completed pre-lab questions are due at the beginning of your lab period. They will count towards your grade on this lab. 3. This is probably the only lab experiment of the year in which you will not have to wear any safety equipment. Enjoy! 4. Record all data in pen on the Data Table contained in this packet. 5. A table of electronegativities from your textbook is included in this packet. You will need it to fill out the Data Table. 6. I will explain the color codes for each element at the beginning of lab. Instructions for Writing your Lab Report1. This will NOT be a formal lab report. Instead, complete the followinga) Title b) Objectives c) Materials d) Procedure e) Place the completed Data Table from this packet in your report. Remember to number it correctly! f) Lewis structures for the molecules shown on the procedure page. g) Answers to post-lab questions. 2. Your lab report is due on . Jack M. Barrack Hebrew Academy 2013 – 2014, Tri 2 Honors Chemistry Dr. Darin Katz Lab Experiment #5- Molecular Models and Polarity Pre-lab Questions Name Please answer the following questions after reading your lab sheets. These completed questions are due at the beginning of your lab period. 1. What is the absolute determining factor that governs what type of bond will form between two atoms? 2. What types of elements combine to form a covalent bond? 3. Why do atoms combine to form a chemical bond? 4. How are the valence electrons arranged differently in an ionic bond vs. a covalent bond? 5. What does it mean when we say that a molecule has “overall polarity”? 6. Now construct a data table with the following 4 columns: (1) Molecule; (2) Bonds in molecule; (3) Electronegativity Difference; (4) Molecular Polarity. Fill in the formula for each of the 11 molecules you will be building in the “Molecule” column. DDK will check the data table before you start the lab. The data table will count for 2 points of your pre-lab grade. Background Information Models are useful in visualizing the three-dimensional structure of molecules. The purpose of this lab is to construct models of some common molecular compounds (i.e., only has covalent bonds) and use these models to determine molecular polarity. The color of each gum drop will indicate the element and the number of toothpicks represents the number of bonds it forms in the molecule. I will explain the color coding to you in lab. A table of electronegativities is provided for you. Procedure 1. Construct each of the following molecules. Follow steps 2 – 4 before disassembling each model. nitrogen (N2) phosphorous trichloride (PCl3) sulfur trioxide (SO3) hydrogen chloride (HCl) hydrogen sulfide (H2S) carbon dioxide (CO2) ammonia (NH3) carbon tetrabromide (CBr4) trichlorofluoromethane (CFCl3) nitrite ion (NO2-) 2. Determine the electronegativity difference for each bond in the molecule. Record this difference in the Data Table. 3. From Step 2, determine the bond type for each bond and record it in the Data Table. 4. Draw the Lewis structure of each molecule shown above. Include this on a separate sheet of paper or put them directly in your lab notebook. 5. Using the bond orientation(s) and the polarity of each bond, determine if the molecule has overall polarity. Post-Lab Questions There is NOT enough room to answer these questions in the space provided. Answer these questions in your lab notebook. Include the question followed by your answer. Type the question and answers and paste them into your lab notebook. 1. Which molecules did you determine to possess overall molecular polarity? 2. Which molecules did you determine to NOT possess overall molecular polarity? 3. Which molecular geometry(s) always produce a molecule with overall polarity? Why is this so? 4. Is it possible for a molecule to possess polar covalent bonds but not have overall molecular polarity? Fully explain your answer. 5. Based on your determination of molecular polarity for each molecule in this lab experiment, identify the dominant intermolecular force (IMF) for each molecule. 6. One of the most important phrases in chemistry is “like dissolves like”. Explain this phrase in terms of polarity of solutes and solvents. You may use internet sources and your textbook to answer this question. Be sure to cite your sources. 7. Why do many molecules dissolve in water, yet others do not? Based on your determination of molecular polarity, identify which molecules from this lab experiment would dissolve in water and which would not. You may use internet sources and your textbook to answer this question. Be sure to cite your sources.