HYDROXY—COMPOUNDS (ALCOHOLS AND PHENOLS)

HYDROXY—COMPOUNDS (ALCOHOLS AND PHENOLS)
I. Introduction
(A) Alcohols
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.1
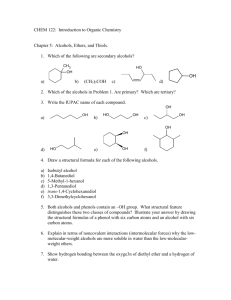
Alcohols are compounds containing one or more hydroxyl groups (—OH) attached to a saturated carbon atom.
The saturated carbon may be a carbon or a simple alkyl group.
Monohydric aliphatic alcohols — alcohols containing one hydroxyl groups.
Examples:
CH
3
CH
3
CH ethanol
2
OH
CH
3
CH
2
CHCH
3
OH
________________________
H
3
C CCH
3
OH
______________________
Polyhydric aliphatic alcohols — alcohols containing two or more hydroxyl groups
Examples:
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH CH
2
OH OH OH OH OH OH OH
__________________ ___________________ _____________________
Note <1> The saturated carbon atom which is attached to the hydroxyl group can be of an alkenyl or alkynyl group.
Examples
CH
2
=CHCH
2
CH
2
OH OHCH
2
C CH ClCH
2
CH=CHOH
___________________ _____________________ ____________________
<2> The carbon atom may be attached to the side chain of a benzene ring.
CH
3
CH
2
OH
CH
3
_____________________
OH
________________________
(B)Phenols (Aromatic alcohols or alcohols)
CH
2
CH
2
OH
_____________________
Phenols are compounds in which the hydroxyl group is directly attached to the benzene ring.
Examples:
Br
OH OH O
2
N OH
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.2
II. Preparation of Alcohols
(A) Hydrolysis of halogenoalkanes through (S
N
) reaction
— to prepare primary and secondary alcohols from a primary and secondary halogenoalkanes.
Mechanism:
OH-
+
CH
2
Br
CH
2
CH
3
Slow H O CH
2
Br
CH
2
CH
3
H O CH
2
CH
2
CH
3
Note: Tertiary alkvl halides undergo ELIMINATION too easily to be of use for synthesizing tertiary lcohols.
(B) Reduction of aldehydes or ketones
— to prepare primary and secondary alcohols from an aldehyde and a ketone (both contain the carbonyl group >C=O
1. Reduction by using hydrogen gas under high pressure with Pt or Ni as catalyst
R
C O
+
H2 high pressure R
CH OH
2.
R R
Reduction by using Lithium tetrahydridoaluminate (LiAlH
4
) which can release hydride ion (H
-
)
H
+
R
R
R
H
+ C O
CH O
CH OH from LiAlH
4
R
R from dil. acid
R
Note : <1> Since LiAlH
4
reacts violently with water, it is necessary to use an inert solvent such as ether (ethoxyethane). Hydrolysis of the intermediate b dilute acid gives the desired
<2> alcohol.
A less powerful reducing agent, sodium tetrahydridoborate (NaBH
4
) can also be used. It is more convenient because it can be used in aqueous or methanolic solution.
R
NaBH
4
R
C O CH OH methanol / water
R R
Example:
1
. LiAlH
4
/ dry ether
CH
3
CH
2
CHO
2
. H
3
O+
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物
1
. LiAlH
4
/ dry ether chapt. 34: p.3
O
2
. H
3
O+
O
1
. LiAlH
4
/ dry ether
HOOCCH
2
C CH
3
2
. H
3
O+
O O
1
. LiAlH
4
/ dry ether
CH
3
O CCH
2
C CH
3
2
. H
3
O+
Note : LiAlH
4
can also reduce carboxylic acid, acid chloride, acid anhydride and esters groups to alcohols.
(C) Hydration of Alkenes
— to prepare secondary and tertiary alcohols from alkenes.
In the presence of acid catalyst, water can be added onto an alkene to form a secondary or a tertiary alcohol (except for ethene) . The reaction reversible and the mechanism is just the reverse of that for dehydration of an alcohol.
Mechanism:
OH
2
H
+
H
H H
C C
+
H
H
H
H H
C C
H O
+
H H
H
H
H H
C C
H OH
H
In practice. the alkene is bubbled into conc. sulphuric acid to form an alkyl hydrogensulphonate.
When this is diluted with water and distilled, an alcohol is formed:
CH
2
=CH
2
+ H
2
SO
4
CH
3
CH
2
HSO
4
CH
3
CH
2
HSO
4
+ H
2
O
CH
3
CH
2
OH + H
2
SO
4
(D) Hydrolysis of Esters with alkali
— to prepare prinary, secondary and tertiary alcohols
Example:
CH
3
COOC
2
H
5
+ NaOH
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物 chapt. 34: p.4
(E) Oxidation of alkenes by alkaline KMnO
4
— to prepare a diol from alkenes.
Example :ethane-1,2-diol can be prepared by bubbling ethene into alkaline potassium manganate(VII) solution.
KMnO
4
/ OH-
H
2
C CH
2
H
2
C CH
2
OH OH
III. Preparation of phenol
(A) By fusion of sodium hydroxide with the sodium salt of benzenesulphonic acid :
SO
2
ONa
+
NaOH
O
+
Na
+
Na
2
SO
3
OH
Phenol is then released from sodium phenoxide with dilute acid:
O Na
+
+ H
+
+ H
2
O
(B) By warming of an aqueous solution of benzene-diazonium chloride
(a common laboratory method)
N
+
N Cl OH
+ N
2
+ H Cl
(C) By the hydrolysis of chlorobenzene with sodium. hydroxide, under he drastic conditions of 150 atm. and at 400
0
C
(an industrial method)
Cl
2 NaOH
O Na
+
H
+ OH
IV. Reactions of Alcohols and Phenols : Basic consideration
Both alcohols and phenols contain the hydroxyl group (—OH) However, as this group is attached to the benzene ring for phenols and to the saturated Carbon for alcohols. the reactions of such compounds are quite different.
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物
(A) Reactions of Alcohols
There are two main types of reactions for alcohols:
1. Reactions involving fission of R—OH bond (or just C—O bond)
2.
C O H alkyl hydroxy fission
Reactions involving fission of RO—H bond (or just O—H bond) chapt. 34: p.5 alkoxy hydrogen fission
C O H
(B) Reactions of phenols
There are two main types of reactions for phenols:
1. Reactions involving the —OH group.
2. Reactions involving the benzene ring.
Note : For phenols. the direct attachment of a hydroxyl group to the benzene ring has mutual effects on the reactivity of both the —OH group and the benzene ring.
The electron—rich benzene ring in phenol can make it undergo electrophilic aromatic substitution.
The reactivity of the —OH group can also be modified by the benzene ring through delocalization effects.
V. Reactions of Alcohols
(A) Reaction involving fission of R—OH bond (cleavage of C—0 bond)
1. Dehydration
(a) intramolecular dehydration (forming alkene)
The conditions for dehydrating alcohols depend closely on the structure of individual alcohols.
<i> For primary alcohols, the conditions required are conc. sulphuric acid a temperature of
170
0
C
CH
3
CH
2
OH
CONC. H
2
SO
4
CH
2
=CH
2
+ H
2
O
170
0
C
<ii> Secondary alcohols dehydrate under milder conditions than primary alcohols.
OH
85 % H
3
PO
4
+ H
2
O
165 – 170 0 C
L.S.T. Leung Chik Wai Memorial School
F.6 Chemistry
Chapter 34: Hydroxy-Compounds 羥基化合物
<iii>Tertiary alcohols dehydrate under even milder conditions.
H
3
C
CH
3
C OH
20 %
H
2
SO
4
H
3
C C CH
2
CH
3
CH
3
Mechanism:
For secondary and tertiary alcohols, the following mechanism is generally accepted. chapt. 34: p.6
C C
+
H
+
H OH
Note : <1> The main function of the acid is to transform the poor leaving group —OH into the very good leaving group. —OH
2
<2> The ease of dehydration of alcohols is
tertiary > secondary > primary
Reason : Tertiary carbocation is the most stable one. i.e. The order of stability of the carbocations follows the number of electron releasing groups
H
3
C
CH
3
C
+
H
3
C
H
C
+
H
H C
+
H
H C
+
CH
3
CH
3
CH
3
H most stable least stable
<3> Dehydration of secondary and tertiary alcohols containing more than three carbon atoms will give a mixture of alkenes whose amounts will be determined by the following rule
Alcohol dehydrations generally produce the more highly substituted alkenes. i.e. the major product is that contains the higher number of alkyl groups attached to the
C=C bond. e.g.
CH
3
CH
OH
CH
2
CH
3
H
+
CH
3
CH
O
+
CH
2
CH
3
-H
2
O
CH
3
C
H
+
CH
2
CH
3
H H
(a) Intermolecular dehydration (forming ether)
When the dehydration is carried out at a temperature of 140
0
C with an excess of alcohol. ether will be formed.
2 CH
3
CH
2
OH
CONC. H
2
SO
4
CH
3
CH
2
OCH
2
CH
3
+ H
2
O
2. Halogenation
Alcohols react with hydrogen halide and phosphorus halides give halogenoalkanes.
(a) Reaction with hydrogen halides
Mechanism:
Step1: Protonation of the alcohols (same process for 1°, 2° and 3° alcohols)
The alcohol acts as a weak base and accept the proton donated by the hydrogen halide.
The equilibrium lies well
ROH
+
H
+
R O
+
H
H
Step 2 : Displacement the halide on for a water molecule.
<i> For primary and secondary alcohols, it is a S
N
2 reaction.
X RCH
2
O
+
H
H
<ii> For tertiary alcohols, it is a S
N
1 reaction.
X
R
3
C O
+
H
H
R
C
+
R R
R
X
R-CH
2
-X
R
C
+
R
R
C
R
R
Note <1> Secondary alcohols also proceed through a mechanism involving the formation of carbocation.
<2> Reactions of primary and secondary alcohols with hydrogen halide are catalysed by zinc chloride in the Lucas reagent. (a solution of ZnCl
2 in conc. HCI).
C
2
H
5
OH
+
H Cl
Reflux
C
2
H
5
Cl
+
H
2
O
ZnCl
2
Catalyst
Chlorination of 2-methylpropan-2-ol in a solution of ZnCl
2 and conc. HCl Example
(i) Mechanism for this reaction : ( a ____ reaction )
(ii) Energy profile for this reaction:
(iii) Rate of the reaction for 1
0
, 2
0
and 3
0
alcohols:
The order of rates of reaction:
3
0
alcohol > 2
0
alcohol > 1
0
alcohol
The rate can be shown by the turbidity in the aqueous layer since the chloroalkane formed is immiscible with water.
(iv) Dependence of the chloride anion concentration.
For tertiary alcohols, the rate is independent of the concentration of chloride ion because it is a S
N
1 reaction.
Example: Bromination of ethanol in a mixture of conc. H
2
SO
4
and solid NaBr conc. H
2
SO
4
/ NaBr
C
2
H
5
OH C
2
H
5
Br +
H
2
O heating
(b) Reaction with phosphorus halides
Alcohols react with phosphorus tribromide and phosphorus tri iodide to form bromo— and iodoalkanes respectively.
3 R-OH
+
PBr
3
3 R-Br
+
H
3
PO
3
3 R-OH
+
PI
3
3 R-I
+
H
3
PO
3
Note :<1>The phosphorus trihalides are prepared by the reaction of red phosphorus and the halogen.
<2> Phosphorus pentahalide or thionlychloride are used to prepare chloroalkanes at room temperature
R-OH + PCl
5
R-Cl + H Cl + POCl
3
R-OH + SOCl
2
R-Cl + SO
2
+ H Cl
Exercise : -
The following apparatus is used to prepare bromoethane (b.p. 38”C) from ethanol; using red phosphorus and bromine.
(a) What advantage is there in using red phosphorus instead of white phosphorus?
(b) What is the purpose of the soda lime tube?
(c) Why is a water condenser included in the set-up.
(d) Explain why a cold water bath is used while bromine is added?
(e) The mixture has to be refluxed for 30 minutes after addition of bromine. Why?
After the heating process the apparatus is converted for distillation and he product is collected in a receiver immersed in cold water. The distillate is then washed with water before drying.
(f) How can you tell when the distillation is complete?
(g) Suggest one impurity that can be removed by washing with water.
(h) Give the name of a drying agent for bromoethane.
(I) What further treatment is required in order to obtain pure bromoethane?
(j) What would you do when setting up the apparatus and reagents, bromine liquid is accidentally split on our hand?
(B) Reaction involving fission of RO—H bond (cleavage of O—H bond)
1. Reaction with active metals (e.g. sodium)
Alcohols are very acids because the alkyl group pushes electrons towards the —OH group, so that the oxygen does not strongly attract the electrons in the —OH bond.
Furthermore. cannot be once a RO
-
ion is formed. it stabilized by the delocalization of the charge. Thus. alcohols react only to a very slight extent with alkalis, but will react wtth very electropositive metals under anhydrous conditions to give alkoxide.
Example: reaction of ethanol with sodium
2CH
3
CH
2
OH + 2Na
2CH
3
CH
2
O
-
Na
+
+ H
2
Addition of water will regenerate the alcohol readily.
CH
3
CH
2
O
-
Na
+
+ H
2
O
CH
3
CH
2
OH + NaOH
Note <1> The reaction is much slower then the reaction of water with sodium.
<2> The reaction of alcohol with sodium can be used to depose the excess sodium in the laboratory.
2. Esterification
Alcohols and carboxylic acids react to give esters.The functional groups of acids and esters are
(where R is an alkyl group)
O O
C C
OH O-R
Carboxylic acid Carboxylic ester
Esterification takes place much faster in the presence of a catalyst such as conc. H
2
SO
4
.
Example :
O conc. H
2
SO
4
CH
3
CH
2
C
OH
+
CH
3
CH
2
OH
Reflux
CH
3
CH
2
C
O
+
OCH
2
CH
3
H
2
O
Alcohols can also react with acid chlorides and acid anhydrides to form esters.
Example:
CH
3
CH
2
C
O
Cl
+
CH
3
CH
2
OH CH
3
CH
2
C
O
+
OCH
2
CH
3
H Cl propanoyl chloride
O
CH
3
CH
2
C
+
O
CH
3
CH
2
C
O propanoic anhydride
CH
3
CH
2
OH CH
3
CH
2
C
O
+
OCH
2
CH
3
CH
3
CH
2
COOH
3. Oxidation
Alcohols can he oxidized by various oxidizing agents to aldehyde, ketones or carboxylic acids. depending on the nature of the alcohol and the strength of the oxidizing agents being used.
Oxidizing agents used:
Acidic or alkaline potassium permanganate, acidified potassium dichromate, chromic acid or dilute nitric acid.
(a) Primary alcohols are readily oxidized through aldehydes to carboxylic acids.
O
O
R-CH
2
OH
C
C
R H
R OH
Primary alcohol
Aldehyde
Carboxylic acid
Note: The alcohol , aldehyde and acid preserve the same number of carbon atoms.
(b) Secondary alcohols are oxidized to ketones under normal conditions
Note
Secondary alcohol Ketone
The ketone formed has to be undergo prolonged drastic treatment before it can be broken down into acids with smaller number of carbon atoms.
OH
CH
3
CH CH
3
O O
CH
3
C CH
3
O O
CH
3
C OH
+
CO
2
(c) Tertiary alcohols are normally resistant to oxidation in the neutral or alkaline medium. because it would involve the breakage of the high energy C—C bonds in the alcohol molecule.
CH
3
H
3
C CH
3
NO reaction
OH
In acidic solutions, tertiary alcohols can he oxidized to give a mixture of ketone and acid, both with fewer carbon atoms than the alcohol.
CH
3 H
3
C
+
H
3
C CH
2
CH
3
O
CH
3
COOH
OH
H
3
C
Note: Characterization of the oxidation products of alcohols is a means of distinguishing between primary , secondary and tertiary alcohols.
<1> The aldehyde may be detected by its reaction with 2,4-dinitrophenylhydrazine. Tollen’s reagent or Schiff’s reagent.
<2> Ketones may be detected by its reaction with 2,4-dinitrophenylhydrazine but not with Tollen’s reagent or Schiff’s reagent.
<3> Carboxylic acids can be detected by reaction with sodium hydrogencarbonate solutions or ester formation.
Reactions of Ethanol, a Typical Aliphatic Alcohol
Ethoxyethane CH
3
CH
2
OCH
2
CH
3
Ethene CH
2
=CH
2
CH
3
CH
2
Cl
PCl
5
/ SOCl
2
Sodium ethoxide CH
3
CH
2
ONa conc. H
2
SO
4
KBr / conc. H
2
SO
4
Na
CH
3
CH
2
Br
C
2
H
5
OH
K
2
Cr
2
O
7
/ H
+
Ethanal CH
3
CHO
CH
3
COCl
I
2
+ red P
OR
KMnO
4
CH
3
COOH H
+
CH
3
CH
2
I
Ethyl ethanoate CH
3
COOCH
2
CH
3
Iodo ethane
Methods of distinguishing between 1 0 , 2 0 and 3 0 alcohol
Reagents Primary alcohol Secondary alcohol
Acidified
K
2
Cr
2
O
7
Conc. H
2
SO
4
Aldehyde,
RCHO formed
Solution change from orange to green
Alkene formed slowly
Ketone,
R
2
CO formed
Solution change from orange to green
Intermediate in speed
Cloudiness appear in
5 minutes
Conc. HCl Cloudiness due to
+
ZnCl
2
Add to alcohol and place formation of RCl is slow to appear. in boiling water bath
Ethanoic acid CH
3
COOH
Tertiary alcohol
No observable change
Alkene formed fast
Cloudiness appears in
1 minute owing to the formation of RCl, which is insoluble in water
Exercise
1. Predict the main product in the following reactions.
(a)
(b)
Primary alcohol added dropwise to acidified potassium permanganate.
Acidified potassium permanganate added dropwise to the primary alcohol
ANSWER
2. (a) Write all possible structural isomers corresponding to the molecular formula C
4
H
10
O
(b) One of these isomers. X. has the following reactions. Deduce the molecular formula of reaction
X from the following reactions. showing what structural information each conveys.
<1> X reacts slowly with sodium metal.
<2> Vigorous oxidation of X yields a product Y, without loss of carbon atoms.
<3> Y reacts with 2,4—dinitrophenylhydrazine to give a yellow crystal but not with sodium metal.
<4> Dehydration of X gives a mixture of hydrocarbon A and B. each containing 85.7% of carbon.
ANSWER
4. Haloform reaction
Alcohols which contain the group
CH
3
C
H
OH can be oxidized under suitable conditions into the
C O group
H
3
C
. This group will readily undergo the haloform reaction:
CH
3
R C
H
OH
NaOH / X
2
RCOONa
+
CH
3
X
2.
Mechanism : The reaction takes place in following three steps.
Oxtdation of alcohol. to an aldehyde or ketone. 1.
Substitution of halogen atoms in the methyl group.
3. Hydrolysis under alkaline conditions to form. the haloform
Note <1> The reaction is usually carried out with warm alkaline solution of halogen (NaOH/X
2
) or a solution of potassium halide and sodium halate(I) (KX/NaOX).
In the Iodoforrrt reactton, the triiodomethane precipitates as yellow crystals. The reaction mechanism can be described as follow:
CH
3 H
3
C
R C
H
OH + I
2
+ NaOH
C O + NaI
+
H
2
O
R
I
2
+ NaOH
RCOONa
+
CH
3
X
<3> Ethanol and 2 alcohols can be distinguished from the other alcohols by using iodoform test because the triiodomethane formed is a yellow crystal.
Exercise
When an alcohol A is refluxed with conc. H
2
SO
4
, at 170°C. compound B is formed as the major product. If B is treated with ozone and then hydrolysed by water. 2 organic products are formed. one is ethanal and the other is propanal. A gives a positive iodoform test. Deduce the structures and give the names of A and B.
ANSWER
VI. Reactions of Phenols
1 Reactions of phenols can he classified into:
(A) those involving the -OH group
(B) those involving the benzene ring attached to it
(A) Reactions of the —OH group
1. Dissociation (Acidic properties — salt formation)
Phenol can dissociate in water
OH
O
+ H
2
O
+ H
3
O+
It behaves as a weak acid because its dissociation occurs to only a light extent
(pKa = 10.0)
Thus, phenol can react with sodium metal
OH O Na
+
+ Na 2
+ H2 2
Unlike alcohol, it can react with NaOH and is a stronger acid than alcohol.
Note : <1> Explanation of the higher acidity of phenol than aliphatic alcohol.
In phenol. the O—H bond breaks more readily and the resultant phenoxide anion is stabilized by resonance.
In the phenoxide ion, a p orbital of the oxygen atom overlaps with the
orbital of the ring carbon atoms.
Therefore. the equilibrium favours dissociation of phenol into H
3
O
+
and the phenoxide ion.
Thus it can be seen that owing to the direct attachment to the benzene ring. the acidity of phenol is greatly enhanced by resonance. and is very different from aliphatic alcohol.
<2> The phenoxide ion formed from the reaction of phenol with alkalis can act as a powerful nucleophile and can be used in the synthesis of certain organic compounds.
OH ONa
+
NaOH + H
2
O
<3> When comparing the acidity with carbonic acid and carboxylic acid, phenol is the weakest.
Methods to distinguish between alcohols, phenols, and carboxylic acid:
(a) Carboxylic acid can liberate CO
2
when treated with sodium hydrogen carbonate solution whereas alcohols and phenols cannot.
(b) Phenols can react with NaOH to give a salt whereas alcohols cannot.
Example: A scheme outlining the chemical reactions of the following compounds A, B and C is listed as below:
OH
CH
3
CH
2
OH CH
3
COOH
A B
C
Mixture of A, B and C in ethoxyethane is shaken with NaHCO
3
solution.
Ethereal layer separate
Aqueous layer
Shaken with aq.
NaOH, then separate
1.
acidify with dil.HCl,
2.
extract with ether again,
3.
evaporate ether
A
Aqueous layer Ethereal layer
C
Add H + aq
, then filter
B
Evapourate ether
2. Other reactions of the –OH group
As stated before, the –OH group of phenol behave differently from those of aliphatic alcohols, as a result of the delocalization of electrons with the benzene ring directly attached to it.
OH
The resonance effect strengthens the C-O bond, the partial double bond character between the carbon atom and the oxygen is confirmed by its bond length being shorter than that of normal
C—O bond.
Thus, Reactions of the —OH group of phenol are quite different from that of alcohol:
(a) Displacement of the —OH group by halogen, pcurs only at more extreme conditions.
(b) Phenol is not oxidized to some breakdown products but form complex polymers.
(c) Phenols does not undergo elimination as primary and secondary alcohol do.
In alkaline medium, phenol generate the phenoxide ion C
6
H
5
O
-
. Such anion is a more powerful nucleophile than /the neutral phenol molecule, and can take place in some nucleophilic reactions.
OH O Na
+
NaOH room temp.
CH
3
CH
2
Br
OCH
2
CH
3
CH
3
COCl
OOCCH
3
O
H
3
C
H
3
C
OOCCH
3
O
O
Note : The reaction between sodium phenoxide and bromoethane is a typical S
N
2 reaction in which the rate depends on the availability of electron pairs at oxygen.
O
CH
3
CH
2
Br
If there is an electron withdrawing group (e.g. —NO group) attached to the benzene ring, the rate will be reduced.
O
N
O
O
(B) Reactions of the benzene ring — Substitution
Phenol is more reactive than benzene towards electrophilic reagents because there is an interaction between the lone pairs on the oxygen atom in —OH or —O and the ring; which increase the availability of electrons in the aromatic ring.
Note : The greater activity of the ring in phenol than in the simple benzene molecule is reflected in the milder
1. Nitration
Monosubstituted compound is obtained with dilute nitric acid at room temperature.
OH dil. HNO
3
OH
OH
NO
2
+
NO
2
If conc. nitric acid is used. trisubstituted product is obtained readily.
OH
OH dil. HNO
3
O
2
N
NO
2
NO
2
2. Halogenation (Bromination)
(a) Chlorine, in the absence of solvent, gives 2— and 4—chlorophenol.
(b) Bromine, in a non—polar solvent (e.g. CS
2 or CCl
4
) gives 2— ,4—bromophenol.
OH
OH OH
CS
2
Br
+
Br
2
+
(c) Bromine water gives a precipitate of 2,4,6—tribromophenol.
Br
The faster reaction in water is due to the presence of phenoxide ions.
OH
OH
Br Br
+
Br
2
Br
3. Sulphonation
When phenol is treated with concentrated sulphuric acid. different substituted products will result, depending on the reaction temperature.
OH
OH SO
3
H
OH
SO
3
H
Exercise
Arrange the following compound in order of increasing rate of reaction with concentrated sulphuric acid. Give reasons for your order.
OH
OH
CH
3
VII. Alkanediols
Alkanediols are examples of polyhydric alcohols which contain more than one —OH group in their structures.
Example 1 : Ethane-1,2-diol (ethylene glycol)
(A) Preparation
Ethene is bubbled into alkaline solution of potassium manganate(VII), the purple fades out as ethene ids oxidized to ethane—1 . 2—diol.
MnO
4
-
H
+
CH
2
=CH
2
CH
2
CH
2
OH OH
A brown suspension of manganese (IV) oxide also appears.
(B) Properties
The properties are similar to those of monohydric alcohols.
(C) Uses 1
It as used antifreeze in car radiators and as a radiators and as a de-icing fluid on aeroplane wings.
Note : Alkanetriols Propane—1.2,3— triol (glycerol)
It is the by—product in the manufacture of soap. Glycerol is used for the manufacture of ester which it forms from nitric acid, propane—1,2.3—triyl trinitrate.
CH
2
OH
CH
2
O-NO
2
CH OH + 3 HNO
3
CH O-NO
2
+
3 H
2
O
CH
2
OH glycerol 甘油
CH
2
O-NO
2 propane-1,2,3-triyl trinitrate 硝化甘油
This ester is also called nitroglycerine. It is used as both explosivee and a drug in medicine to treat heart diseases.
OCH
2
CH
3
SO
3
Na
CH
3
CH
2
Br
NaOH
Br
O
Br
OCOCH
3
Br
Br
2
O Na
+ (CH
3
CO)
2
O
NaOH
Cl
O
2
N
OH
NO
2
Conc. HNO
3
OH
NO
2
HNO
3
OH-
OH
H
+
NO
2
Conc. H
2
SO
4
OH
H
2
O
N
2
Cl
Diazonium salt
SO
3
H
Cl
2
In FeCl
3
FeCl
3
OH
Violet colour (Test for a phenol)
Soluble in NaOH, but it does not react with NaHCO
3
to give CO
2
Cl
Reaction of Phenol