management of infectious waste materials in the laboratories
advertisement

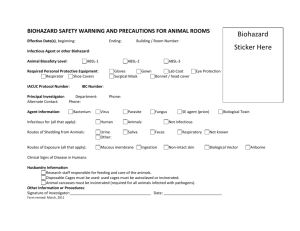
MANAGEMENT OF INFECTIOUS WASTE MATERIALS IN THE LABORATORIES PROTOCOL FOR “SEASONED” AND RECENTLY RECRUITED INVESTIGATORS 1.0 INTRODUCTION As part of the developmental processes of this institution, new investigators may be recruited periodically to perform research activities in the laboratories. This protocol is an attempt to familiarize new and “seasoned” investigators with institutional procedures and processes in the handling, storage, use and disposition of potentially infectious waste. Investigators are reminded that they have certain responsibilities to comply with institutional guidelines. Therefore investigators are advised to familiarize themselves with the Waste Management Manual and other specific protocols pertaining to the management of infectious, hazardous chemicals and radioactive waste substances. 1.1 Research Communication Process for Investigators Investigators who have been conducting research at this institution for more than a year and whose research is funded through one of the NIH programs (i.e., MBRS, MARC, RCMI), should communicate with the program director of each program so that they can distribute copies of the waste management manual to each investigator. Investigators are asked to familiarize themselves with the procedures for waste disposition. In addition, investigators can make an appointment with the Office of Research Operations. Staff members in this office shall provide technical and management information that can be useful in managing specific waste products. Furthermore, Research Operations, in collaboration with the Institutional Biohazard Committee shall provide training workshops pertaining to the management of hazardous materials. 1.2 Research Procedures for Processing Potentially Infectious Waste 1.2.1 The investigator shall be assigned a laboratory through the Institutional Research Space Committee. 1.2.2 The investigator, once assigned a laboratory shall be provided with an infectious waste log form which must be completed by all investigators (refer to copy). Staff in the Office of Research Operations shall distribute the infectious waste log form. Instructions for completion shall be provided. 1.2.3 1.2.4 The generation of any potentially infectious waste must be placed in RED bags, marked infectious waste each day. The completion of the infectious log form must also be completed the same day. 1.2.5 The Biohazard Technician for infectious waste shall retrieve the infectious waste log form each day before the collection of each “red” bag(s). 1.2.6 On satisfactory completion of the above form, the technician shall submit this form to the Research Operations Office. 1.2.7 The Biohazard Technician shall then collect each red bag, only when the red bags are securely tied, autoclave tape placed on the red bags and the infectious waste log form is completed. 1.2.8 The Biohazard Technician shall collect the “red” bags from each lab and place them in the “BFI grey containers marked unprocessed infectious waste”. 1.2.9 The Biohazard Technician shall process potentially infectious waste on a daily basis. The records for each “cycle” must be kept on file. 1.2.10 At the end of each cycle, the Biohazard Technician shall place autoclaved waste in specific containers awaiting the final collection by an outside vendor. 1.3 1.4 Research Procedures for Utilizing “Sharp Containers” 1.3.1 “Sharp containers” shall be provided by the Research Operations Office for the sole purpose of collecting sharp objects (i.e. needles, syringes, etc.) 1.3.2 The Biohazard Technician shall distribute and keep records of this distribution to each laboratory that requires these containers. 1.3.3 The investigator shall request from the Biohazard Technician for infectious waste when the need arises for additional “sharp containers”. 1.3.4 The Biohazard Technician for infectious waste shall retrieve all “sharp containers” once they are completely filled. 1.3.5 The Biohazard Technician shall place these containers in the appropriate “vendor” containers for final collection by the outside vendor. 1.3.6 Special containers for glassware that are not potentially infectious shall be provided. Termination or Completion of Research Program 1.4.1 On completion of a research program, investigators can remind their program director that there will be a necessity for “close out” of this research program. 1.4.2 The investigator or program director can inform the Research Operations Office. 1.4.3 The Office of Research Operations shall instruct the Biohazard Technician for infectious waste to retrieve all infectious waste log forms and red bags for processing. 1.5 1.6 1.4.4 All “sharp containers” shall be removed by the Biohazard Technician and processed according to institutional procedures. 1.4.5 The Biohazard Technician shall conduct infectious materials surveillance on all “close out” research. 1.4.6 Once this surveillance has been completed, a report shall be completed by Research Operations. 1.4.7 A final report shall be completed and submitted to the Biohazard and Safety Committee for approval. 1.4.8 Once approved by the institutional committee, the investigator of record on the “close out” program shall sign the report, signifying finality of this research program. 1.4.9 This record shall be kept on file in the Office of Research Operations. Responsibilities of the Research Investigator 1.5.1 It is the responsibility of each investigator to ensure that all laboratory research personnel comply with this policy and the policy of the waste management manual. 1.5.2 Investigators are to familiarize themselves with all institutional biohazard protocol. 1.5.3 Investigators and research staff shall also attend workshop and training sessions conducted by the Office of Research Operations in collaboration with the Institutional Biohazard and Safety Committee. Responsibilities of the Biohazard Technician for Infectious Waste 1.6.1 The Biohazard Technician shall maintain separate and service autoclave equipment. 1.6.2 The technician shall handle, store, retrieve and process infectious waste. 1.6.3 The technician shall determine the appropriateness of packaging infectious materials.