Testing Facility - Chhattisgarh Council Of Science & Technology
advertisement

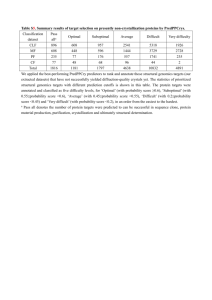
CENTRAL LABORATORY FACILITY (CLF) GUIDELINES FOR TESTING FACILITY UV – Vis Spectrophotometer UV – Vis – NIR Spectrophotometer Atomic Absorption Spectrophotometer Inductively Coupled Plasma Mass Spectrophotometer Central Laboratory Facility (CLF) CLF is envisaged to inculcate and induct scientific temperament and innovativeness in the scientific community, academia and students of the state. CLF will not only provide the state of art instrumentation facility to the scientists of the state but will also help in percolation of the new innovation to the grass root level by facilitating issue based research in the priority areas of the state’s development, imparting instrumentation training to the budding researchers, providing quality assessment facility to the industries of the state and documentation of the new innovations nationally and internationally. Objectives Domains of CLF encompass the following: Serve as an incubator for specific research programmes by providing space, funds and facility to scientists of excellence nationally and international. To provide state of the art instrumentation facility. To cultivate the instrumentation culture in the scientific community in the state. Support/undertake research, design and developmental projects. To attract high level resources expertise by providing R&D opportunities which would result in massive enrichment in scientific culture apart from economic gains. To ensure capacity building in various faculties of science and technology by imparting training to scientists, researchers, academicians and entrepreneurs. Guidelines: CLF in accordance with its customary domains will extend testing facility in various fields such as: Agriculture, Archeology, Bioenergy, Biotechnology, Chemical Sciences, Earth Sciences & Geology, Environmental Sciences, Life Sciences Material Sciences, Medical Sciences, Medicinal & Aromatic Plants, Pharmaceuticals (Phytochemical/Herbal Industry), Physical Sciences, Other areas/fields/sectors relevant to the state. 2. Under the CLF the major analysis techniques available presently for R&D and testing work are: Spectroscopy: UV-Vis, NIR, FTIR, Flame Spectroscopy, Mass Spectroscopy; Chromatography: Gas Chromatography, Liquid 1. Gas Chromatograph – Mass Spectrophotometer CHHATTISGARH COUNCIL OF SCIENCE AND TECHNOLOGY LOKASH PLAZA, SHANKAR NAGAR, RAIPUR 3. i. ii. iii. iv. Chromatography, Layer Chromatography, and Microwave sample preparation system and Microwave Sample Preparation System. The testing work can be taken up on these equipments under the umbrella of CLF for industrialists and others on the following basis: Samples have to be delivered at the Central Laboratory Facility. Samples should be packaged in the following manner: a. Primary receptacle: A labelled primary water tight, leak-proof receptacle containing the specimen. The receptacle is wrapped in enough absorbent material to absorb all fluid in case of breakage. b. Secondary receptacle: A second durable, water tight, leak-proof receptacle to enclose and protect the primary receptacle(s). Several wrapped primary receptacles may be placed in one secondary receptacle. Sufficient additional absorbent material must be used to cushion multiple primary receptacles. c. Outer shipping package: The secondary receptacle is placed in an outer shipping package which protects it and its contents from outside influences such as physical damage and water while in transit. Hand carriage of infectious substances is strictly prohibited. The prerequisites of the testing to be done should be properly labeled along with the following details: a. Pathogenicity of the agent and infectious dose in case of any infectious agent or compound. b. Potential outcome of exposure. c. Natural route of infection. d. Other routes of infection, resulting from laboratory manipulations (parenteral, airborne, ingestion). e. Stability of the agent in the environment. f. Concentration of the agent and volume of concentrated material to be manipulated. g. Information available from animal studies and reports of laboratoryacquired infections or clinical reports if any. h. Laboratory activity planned (sonication, aerosolization, centrifugation, etc.). i. Any genetic manipulation of the organism that may extend the host range of the agent or alter the agent’s sensitivity to known, effective treatment regimens. j. Local availability of effective prophylaxis or therapeutic interventions. Standard precautions necessary should always be mentioned in handling the sample and proper indication of precaution including barrier protections needed must be (gloves, gowns, eye protection etc.) mentioned whenever needed. v. Safety/Biosafety level should be indicated on the sample as applicable. vi. Testing of nuclear material, radioactive material, radioactivity, explosives and explosive substances would not be tested under Central laboratory Facility. 4. The intender should provide details of the history of each sample like- objective of the testing, place of origin, method of collection, date and time of collection, Elements/compounds to be tested for (if unknown possible Elements/compounds should necessarily be indicated), type of testing required, possible outcome of testing. The intender should also provide previous (if any) testing results carried out either by analytical methods, with names of equipments and type of test and method used and place of testing. 5. The intender should declare – how the results of the testing would be used and how would the results be useful for his said objectives. 6. TESTING COST: Testing cost for Individual and Sponsored Researcher/Academician/Scientist is as follows: Name of equipment Number of tests Fees Advance Instrumentation 5 to 10 Rs. 2500/- * (As in point 2) 10 to 20 Rs. 2000/- * 20 to 50 Rs. 1500/- * > 50 Rs. 1000/- * Other subsidiary Depending upon Rs. 10000/equipments space, usage etc. (One time for 2 months) * Rates/per sample 7. Reports of the testing would be provided in hard copy. 8. Duration of testing (time period for testing and report generation and delivery) would be intimated in the testing acceptance letter. 9. Permission for availing testing facility in Central Laboratory Facility remains the prerogative of Director General, Chhattisgarh Council of Science and Technology. 10. Decision of Director General, Chhattisgarh Council of Science and Technology remains final in any/all cases. Contact Chhattisgarh Council of Science and Technology Lokash Plaza, First Floor, Shankar Nagar, Raipur (C.G.) 492 007 Phone: 0771- 2444513; Fax: 0771-2434093