CHEM 2212 Organic Chemistry II Summer 2008 Laboratory Program
advertisement

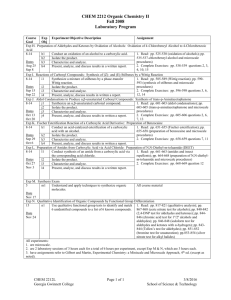
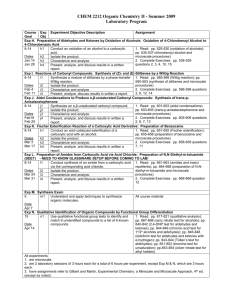
CHEM 2212 Organic Chemistry II Summer 2008 Laboratory Program Course Exp Experiment Objective Description Assignment Goal Obj Exp H. Preparation of Aldehydes and Ketones by Oxidation of Alcohols: Oxidation of 4-Chlorobenzyl Alcohol to 4-Chlorobenzoic Acid 8-14 h1 Conduct an oxidation of an alcohol to a carboxylic acid. 1. Read: pp. 525-530 (oxidation of alcohols); pp. 535-537-chlorobenzyl alcohol and microscale h2 Isolate the product. Dates procedures) h3 Characterize and analyze. Jun 24 2. Complete Exercises: pp. 538-539 questions 2, 3, h4 Present, analyze, and discuss results in a written report. Jun 25 4, 10, 15 Exp I. Reactions of Carbonyl Compounds: Synthesis of (Z)- and (E)-Stilbenes by a Wittig Reaction 8-14 i1 Synthesize a mixture of stilbenes by a phase-transfer 1. Read: pp. 585-589 (Wittig reaction); pp. 590Wittig reaction. 593 (synthesis of stilbenes and microscale Dates procedures) i2 Isolate the product. Jun 30 2. Complete Exercises: pp. 596-598 questions 3, 6, i3 Characterize and analyze. Jul 1 12, 14 i4 Present, analyze, discuss results in written a report. Exp J. Aldol Condensations to Produce α,β-unsaturated Carbonyl Compounds: Synthesis of trans-p-Anisalacetophenone 8-14 j1 Synthesize an α,β-unsaturated carbonyl compound. 1. Read: pp. 601-603 (aldol condensations); pp. 603-605 (trans-p-anisalacetophenone and microscale j2 Isolate the product. Dates procedures) j3 Characterize and analyze. Jul 2 2. Complete Exercises: pp. 605-606 questions 2, 6, j4 Present, analyze, and discuss results in written a report. Jul 7 7, 13 Exp K. Fischer Esterification Reaction of a Carboxylic Acid Derivative: Preparation of Benzocaine 8-14 k1 Conduct an acid-catalyzed esterification of a carboxylic 1. Read: pp. 651-655 (Fischer esterification); pp. acid with an alcohol. 655-658 (preparation of benzocaine and microscale Dates procedures) k2 Isolate the product. Jul 8 2. Complete Exercises: pp. 658-659 questions 7, 11 k3 Characterize and analyze. Jul 9 k4 Present, analyze, and discuss results in written a report. Exp L. Preparation of Amides from Carboxylic Acid via Acid Chloride: Preparation of N,N-Diethyl-m-toluamide (DEET) 8-14 l1 Conduct synthesis of an amide from a carboxylic acid via 1. Read: pp. 661-663 (amides and insect the corresponding acid chloride. repellents); pp. 664-668 (preparation of N,N-diethylDates m-toluamide and microscale procedures) l2 Isolate the product. Jul 14 2. Complete Exercises: pp. 668-669 question 12 l3 Characterize and analyze. Jul 15 l4 Present, analyze, and discuss results in a written report. Exp M. Synthesis Exam 5 m1 Understand and apply techniques to synthesize organic All course material molecules. Date Jul 16 Exp N. Qualitative Identification of Organic Compounds by Functional Group Differentiation 15 n1 Use qualitative functional group tests to identify and match 1. Read: pp. 817-821 (qualitative analysis); pp. 6 unidentified compounds to a list of 6 known compounds. 867-868 (ceric nitrate test for alcohols); pp. 840-842 Date (2,4-DNP test for aldehydes and ketones); pp. 844Jul 21 846 (chromic acid test for 1o/2o alcohols and aldehydes); pp. 846-848 (iodoform test for aldehydes and ketones with α-hydrogen); pp. 843844 (Tollen’s test for aldehydes); pp. 851-852 (bromine test for unsaturation); pp.853-854 (silver nitrate test for alkyl halides) All experiments: 1. are microscale. 2. are 2 laboratory sessions of 3 hours each for a total of 6 hours per experiment, except Exp M & N, which are 3 hours each. 3. have assignments refer to Gilbert and Martin, Experimental Chemistry: a Miniscale and Microscale Approach, 4 th ed. (except as noted). CHEM 2212L Georgia Gwinnett College Page 1 of 1 7/16/2016 School of Science & Technology