USDA-KSU ABI3730 Protocol
advertisement

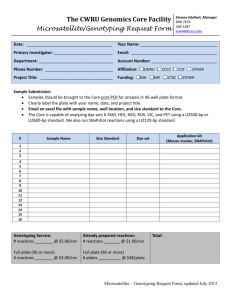
Tuesday, November 18, 2014. St. Amand Page 1 of 12 Genotyping Lab ABI Protocols Overview The ABI 3730 is a 48 capillary electrophoresis system that uses fluorescently labeled dyes for detection of DNA. Typically, a PCR reaction that will use the ABI will have 3 primers in the PCR mix: a "tailed" forward primer, a reverse primer, and an M13 Dye labeled forward primer. Conversely, primers may be "directly labeled" and in that case, only two primers are used just like a standard PCR. Up to 4 dyes (4 different PCR reactions) can be pooled into a single ABI capillary and run at the same time. Primers Modify the FORWARD primer sequence by adding an "M13" tail to the 5' end of the primer. We use an 18 base tail. For example: 5'- ACG ACG TTG TAA AAC GAC + primer sequence -3' Do not modify the reverse sequence. Resuspend new lyophilized primers in Te (10mM TRIS, 1mM EDTA) to a stock concentration of 100 pm/ul and working concentration of 10 pm/ul. Most of the SSR primers in our lab are already "tailed". Before using a primer, find out if it is tailed or not. M13 primers are labeled with fluorescent dyes. Dye Set "G5" FAM (Blue) VIC (Green) NED (Yellow) PET (Red) 5'5'5'5'- FAM-ACG VIC-ACG NED-ACG PET-ACG ACG ACG ACG ACG TTG TTG TTG TTG TAA TAA TAA TAA AAC AAC AAC AAC GAC GAC GAC GAC -3' -3' -3' -3' Dye Set "D" FAM (Blue) 5'- FAM-ACG ACG TTG TAA AAC GAC -3' HEX (Green) 5'- HEX-ACG ACG TTG TAA AAC GAC -3' NED (Yellow) 5'- NED-ACG ACG TTG TAA AAC GAC -3' We keep dye labeled M13 primers in stock in TH3401. Certain dyes can be substituted for others with fair results. VIC and HEX are interchangeable. NED and TAM are interchangeable. Use these same dye sets if you are directly labeling a primer. VIC, NED, and PET dye labeled Oligos must be ordered from https://www.lifetechnologies.com/order/custom-oligo/fluorescent-labeledprimers. FAM can also be ordered there or from any other oligo maker. Tuesday, November 18, 2014. St. Amand Page 2 of 12 PCR The following master mix works well for most SSR and STS primers, but may need to be modified for specific primers. Tween-20 at a final concentration of 0.5% is often helpful. Create a master mix by adding the following reagents (in this order): Reagent (Stock Conc.) Autoclaved Nanopure Water Ammonium Sulfate Buffer Stock (10.00 X) MgCl2 (25.00 mM) dNTP mix (5.00 mM each) Forward Tailed Primer (10.00 uM) Reverse Primer (10.00 uM) M13 Dye labeled Primer (10.00 uM) Home-made Polymerase (5.00 U/ul) Master Mix total = DNA (10 to 100 ng/reaction) = PCR reaction total volume = Vol (ul/reaction) 6.472 1.300 1.300 0.520 0.065 0.104 0.039 0.200 10.00 3.00 13.00 Final Conc. 1X 2.50 mM 200.00 uM each 50.00 nM 80.00 nM 30.00 nM 1.00 U/Rxn Thermocycler profiles The following profiles work well for SSR primers and many STS primers, but may need to be modified for specific primers. The Q1SSR profile differs from the LicorSSR profile only in step 11 and in denaturing, but is about 45 minutes quicker per run. That allows the same PCR block to be used 4 to 5 times per day as opposed to 2 or 3 times per day. The F50SSR profile takes about as long as Q2SSR but has more final cycles for increased amplification. LicorSSR (Calculated profile, ~4 hours per run) 1. 95C, 5 min 2. 95C, 45 sec 3. 68C, 5 min, -2.0C/cycle 4. 72C, 1 min 5. Goto step 2, 4 more times 6. 95C, 45 sec 7. 58C, 2 min, -2.0C/cycle 8. 72C, 1 min 9. Goto step 6, 4 more times 10. 95C, 45 sec 11. 50C, 2 min 12. 72C, 1 min 13. Goto step 10, 24 more times 14. 72C, 5 min 15. 4C, 15 min Q2SSR (Quicker LicorSSR, Calculated profile, ~3 hours per run) 1. 95C, 5 min 2. 96C, 1 min 3. 68C, 5 min, -2.0C/cycle 4. 72C, 1 min 5. Goto step 2, 4 more times 6. 96C, 1 min 7. 58C, 2 min, -2.0C/cycle 8. 72C, 1 min 9. Goto step 6, 4 more times 10. 96C, 1 min 11. 50C, 1 min 12. 72C, 1 min 13. Goto step 10, 24 more times 14. 72C, 5 min 15. 4C, 5 min F50SSR (Calculated profile, 3:13h) Tuesday, November 18, 2014. St. Amand 1. 95C, 5 min 2. 96C, 1 min 3. 68C, 3 min, -2.0C/cycle 4. 72C, 1 min 5. Goto step 2, 4 more times 6. 96C, 1 min 7. 58C, 2 min, -2.0C/cycle 8. 72C, 1 min 9. Goto step 6, 4 more times 10. 96C, 20 sec 11. 50C, 20 sec 12. 72C, 30 sec 13. Goto step 10, 39 more times 14. 72C, 5 min 15. 4C, 5 min Page 3 of 12 Tuesday, November 18, 2014. St. Amand Page 4 of 12 Pooling PCR Reactions Up to 4 separate PCR reactions can be pooled into one ABI sample if each PCR uses a different M13 dye. Pooling is not necessary but greatly increases ABI throughput and lowers costs. We use dye set "G5" which has 4 dyes for samples and a 5th dye (LIZ) for the standard. Each of the 4 sample dyes will fluoresce at different wavelengths (colors) and at different intensities. Intensity differences require specific pooling ratios for the samples. Robot scripts are available for pooling using 384 and 96 well plates. We can also use dye set "D" which has 3 dyes for samples and a 4th dye (ROX) for the standard. For dye set "G5", vortex and centrifuge each PCR plate and add: 3.0 ul FAM, 2.5 ul VIC (or HEX), 3.5 ul NED (or TAM), & 3.5 ul PET into 7.5 ul water. Keep the total volume of the pool at 20 ul even if you are not pooling all 4 colors. For dye set "D", vortex and centrifuge each PCR plate and add: 3.0 ul FAM: 3.0 ul HEX (or VIC): 4.0 ul NED (or TAM) into 10 ul water. Keep the total volume of the pool at 20 ul even if you are not pooling all 3 colors. The pooled plate can be stored in the dark at -20°C until needed, but avoid freeze-thaw cycles. Preparing Samples for an ABI run A size standard must be added to each sample. The standard is labeled with an orange dye (LIZ) for dye set "G5" or a red dye (ROX) for dye set "D". The samples must also be suspended in formamide to denature the DNA. Prepare the formamide-standard (FS) mix on ice. Add 0.15 ul GS500 LIZ (or ROX) to 9.85 ul Hi-Di Formamide per sample, vortex. For CASSUL600, use 0.075 ul per sample (or a ratio of 0.06, STD:Sample). For CASSUL1200, use 0.10 ul per sample (or a ratio of 0.08, STD:Sample). (ALWAYS examine the peak heights of your size standard. It should never be below 200 RFU. It should average about 400 RFU. If it is above 800 RFU, you are wasting size standard and you are LOWERING the sample peak heights because of competition between the standard and your sample.) Pipette 10 ul of the FS into each well of a new, empty plate. Vortex and centrifuge the pooled plate. Add 2.0 ul from the pooled plate to the FS plate for a total volume of 12.0 ul per sample. Once the sample is mixed with formamide, it is best to run the plate on the ABI within 48 hours. However, we have frozen plates at -80 for a week or two and found them to be fine. Denature the FS plate at 95°C for 5 minutes, place on ice for 5 minutes, centrifuge, and then place an ABI rubber septa on the FS plate. Load the plate into a carrier and place it in the ABI 3730. Purchase size standards and Hi-Di Formamide from Lifetechnologies (www.lifetechnologies.com). Get ABI3730 certified plates from LabSource and MidSci. Tuesday, November 18, 2014. St. Amand GeneScan™ 400HD ROX™ Size Standard GeneScan™ 500 LIZ™ Size Standard GeneScan™ 500 ROX™ Size Standard GeneScan™ 600 LIZ™ Size Standard GeneScan™ 1200 LIZ™ Size Standard Hi-Di™ Formamide ABI3730 96 well plates, www.clpdirect.com ABI3730 96 well plates, LabSource ABI3730 384 well plates, MidSci ABI3730 96 well plates, bioexpress.com Page 5 of 12 Part #402985 Part #4322682 Part #401734 Part #4366589 Part #4379950 Part #4311320 Part #3442 Part #T53-407 Part #AV384 Part #T-3149-1 User profiles on the ABI 3730 Before any runs can be started, each user must have their own user profile (results group) stored in the ABI software. Ask for help in setting up your own results group on the lab computer. This only needs to be done once. Plate Records Prepare a new plate record for each plate to be run. Records differ slightly for 96 vs. 384 well plates. Get an example plate record from Paul or Amy. The "container name" must be a new, unique name or the record will fail to import. The plate record stores the name of each sample, the instrument protocol, and the well position of each sample. See the example below. Sample names can not contain spaces, tabs, periods, slashes, or unusual characters. Use Excel or a word processor to prepare the plate record and save the file as a tab-delimited textonly file. Use your own name in the "owner" and "results group" fields. The "instrument protocol" must be listed exactly as "gene_mapper_50_DS-33-600bp" for dye set "G5" or "gene_mapper_50_DS-30" for dye set "D". For longer sized fragments, use the G5 dye set and "gene_mapper_50_DS-33-1200bp". From the "Plate Record" section of the ABI software, import your plate record. Container Name Plate ID PlateNameHere PlateNameHere AppServer AppInstance GeneMapper GeneMapper_Generic_Instance Well Sample Name A01 Description ContainerType AppType Owner Operator PlateSealing 96-Well Regular Paul Paul Septa Size Standard Results Group 1 Instrument Protocol 1 Sample1 CASSUL5 Paul gene_mapper_50_DS-30 B01 Sample2 CASSUL5 Paul gene_mapper_50_DS-30 C01 Sample3 CASSUL5 Paul gene_mapper_50_DS-30 D01 Sample4 CASSUL5 Paul gene_mapper_50_DS-30 E01 Sample5 CASSUL5 Paul gene_mapper_50_DS-30 F01 Sample6 CASSUL5 Paul gene_mapper_50_DS-30 G01 Sample7 CASSUL5 Paul gene_mapper_50_DS-30 H01 Sample8 CASSUL5 Paul gene_mapper_50_DS-30 A02 Sample9 CASSUL5 Paul gene_mapper_50_DS-30 B02 Sample10 CASSUL5 Paul gene_mapper_50_DS-30 … … … … … H12 SampleN CASSUL5 Paul gene_mapper_50_DS-30 SchedulingPref 1234 Tuesday, November 18, 2014. St. Amand Page 6 of 12 Data Files The data for each individual well in the plate will be output as a separate file. For example, a 384 well plate will create 384 separate files. Each file contains the data for all 5 dye colors for a given well. The files are named using the sample name (as it appears in the plate record), the well position, the capillary, and the run number. All files are placed on drive E:/ in a new folder named with the date and the "container name" as listed in the plate record. It is very helpful to name samples using the plant name, the primer used, and the dye or color used for each primer. Tuesday, November 18, 2014. St. Amand Page 7 of 12 Runs and Well positions The array has 48 capillaries. For a 96-well plate, injections are made from every well in a column and skips 1 column. A full 96-well plate requires 2 runs (loads) to inject all samples. See figure. A minimum number of samples in a plate is 48 and must be loaded in odd columns only. If you have fewer samples than 48, put ddH20 in the empty wells. For a 384-well plate, injections are made from every other well in a column and skips 3 columns. See figure. A full plate of 384 sample requires 8 runs (loads) to inject all samples once. A minimum number of samples in a plate is 48 and must be loaded as below in the "First Quadrant, First Load" pattern. If you have fewer samples than 48, put ddH20 in the empty wells. Tuesday, November 18, 2014. St. Amand Page 8 of 12 Tuesday, November 18, 2014. St. Amand Page 9 of 12 Starting an ABI Run Do not load a plate or plate record without assistance from either Amy or Paul. Place your samples in the proper size carrier and place the carriers on the tray. Insure that the carriers are fully seated and that all septa are in place. Close the ABI tray stacker. In the "Run Scheduler" panel of the ABI software, press the "Find All" button to locate your plate record. Click on the plate record to select it then click on "Add". The run can then be started. A 96-well genotyping plate takes 1.5 hours to run. A 384-well plate takes 6 hours to run. A 384-well plate with GS1200 or CASSUL1200 takes 12 hours to run. A plate must be run within 48 hours once the sample is added to the formamide. Errors or Problems If the ABI stops during a run and presents an error message, use the "Print Screen" button on the keyboard to copy the screen. Open the "Paint" application and paste the graphic image in the file and save the file to the desktop. That way, we can send the exact error message to the ABI technicians. You should then alert Paul or Amy to the problem. If it is necessary to restart the computer or the ABI, the proper procedure is: 1. Quit all 4 programs of the Data Collection Software 2. Shut down the computer 3. Press the "On" button on the front of the ABI to switch it off. 4. Restart or reboot the PC. 5. Log-in as "Administrator" with the password "WheatDNA" 6. Once log-in is complete, press the "On" button on the ABI. 7. Once the ABI has started and is showing a steady green light, start the "Data Collection" Software. Data Backup All of your run data will be stored in your own run folder. It is YOUR responsibility to copy each batch of data to your own folder on the back-up drive (H:/). Your data may also be emailed to you. You should also copy the data to a portable USB key drive and move it to another computer. Do NOT store data on the computer connected to the ABI 3730. For safety reasons, the ABI 3730 is NOT connected to the network or to the Internet. Do your data analysis on another computer, not on the ABI 3730. Do not quit the ABI Data Collection Software. Do not run any other software on the ABI 3730. Do not install any other software on the ABI 3730. Do not insert disks or USB devices in the ABI computer. ABI Maintenance ABI maintenance is done by Amy or Paul. Once a week the PC and the ABI should be rebooted, the buffer and water changed, and the water jacket flushed. Once a month, the polymer bottle should be replaced with new POP-7. Every 500 to 1000 runs, the array should be changed. Every 4 to 6 months the air filter Tuesday, November 18, 2014. St. Amand Page 10 of 12 should be changed. If specific capillaries seem to be plugged, have high background florescence, or lower signal intensity, then the capillary likely should be changed. Every capillary change requires that a spatial calibration and a matrix calibration be done. Tuesday, November 18, 2014. St. Amand Page 11 of 12 Data Analysis We use "Gene Marker" software to analyze ABI data. This software can be installed on any computer, but requires a USB license key in order to run. We own 5 keys. The basic steps required to use this software are: 1. Add data files (File menu > Open Data). Add only those files produced using the same set of primers. 2. Process files (Project menu > Run). Select "ABI-5-Color" template, the appropriate size standard (usually GS500 or CASSUL), and "Fragment Analysis(Plant)". Press the "Next" button and insure that the following items are "checked" and on: Auto Range, Smooth, Peak Saturation, Baseline Subtraction, Pull-up Correction, Spike Removal, and Local Southern. The following are useful first-time options and may need to be adjusted for your data: Allele Call start=50bp, end=600bp, Intensity=200, Global Percentage=0, Local Percentage=25, Stutter Peak Filter=on, left=95, right=95. Press the "Next" button, set Reject=0 and Check=0. Then press the "OK" button. 3. Save the project (File menu > Save Project). Save your project now and after other steps because the program will occasionally lock-up and become unresponsive. 4. Check the calibration. From the button bar choose "Size Calibration". Each sample file will have a score. Those above 90 are usually sized properly. You should examine each file and make sure that all of the expected size peaks are properly marked with a green triangle. If they are not, control-click on the triangle and move the triangle to the proper peak. Once all peaks have been adjusted for that file, right-click and choose update calibration. Then move on to other sample files. Close the calibration window and re-save the project. 5. View the data. Double-click on a file to view a trace of the data or press the "Gel Image" button to see a representation of all of the samples. 6. View the report. Press the "Show Report" button to see which peaks were identified. Change report options using the "Report Settings" button and the "Show Color" button. The report only shows the currently selected colors. 7. Change allele calling options. If the report shows peaks that are misidentified you will need to change the allele calling options and "re-call" the alleles. Press the "Call Allele" button. Change the allele range and detection threshold options to increase or decrease the allele calling sensitivity based on your data. You will likely need to do this several times and then examine each peak and manually correct some of the calls. After saving a report and PDF for each completed primer, you will likely need to re-call alleles using different settings for other primers. Re-calling alleles removes all previous calls for all primers. 8. Create a Panel. This is an optional, but usually necessary step. Open the panel editor and create a new panel using the manual options. Create a new marker for each color. Insert new alleles at the desired locations for each marker. Save the panel. You must then "re-call" the alleles using this panel. The report will now Tuesday, November 18, 2014. St. Amand Page 12 of 12 only show defined alleles based on the panel used. You may also need to change the allele calling options to refine called bands. 9. Output a report. Once the report is correct. Press the "Save Report" button to export a text-only version of the report. 10. Print trace files. To save the graphic images of the trace data, choose the printer icon and change the settings and print. We have a PDF printer installed so that you may save PDF versions of the print file. Simply choose "Cute PDF Writer" for the printer model. 11. Save gel image. To save the graphic gel image of the data, press the "Show Gel Image" button and then the small "=>" icon near the image. You can then export a graphic image of the gel. 12. Save your project and create a back-up to another location to prevent dataloss. 13. Evaluating peak data. When using fluorescent detection, the best results are obtained when the allele peak heights are above 1,000 RFU and below 15,000 RFU. Peaks below 1,000 RFU may be usable, but the software has a harder time doing auto scoring and small amounts of DNA contamination can produce background peaks. Peaks below 200 RFU should NOT be used. Peaks larger than 15,000 RFU cause “dye pull-up” problems. This produces a false peak in the other color channels. Also, the software may not score very large peaks well. If your peaks are too large, load less of the pool into the formamide solution. If your peaks are too small, try loading 50% more of the pool and also try loading 50% less of the pool. Too little sample can produce small peaks, but too much sample can also produce small peaks. 14. Evaluating size standard peaks. ALWAYS examine the peak heights of your size standard. It should never be below 100 RFU. It should average about 400 RFU. If it is above 1000 RFU, you are wasting size standard and you are LOWERING the sample peak heights because of competition between the standard and your sample.