SI-apl - AIP FTP Server
advertisement

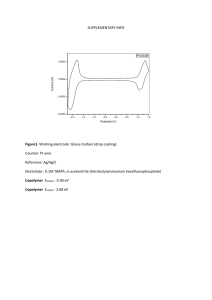
Supplementary Material Carbon quantum dots coated BiVO4 inverse opals for enhanced photoelectrochemical hydrogen generation Feng Nana, Zhenghui Kang*b, Junling Wangc, Mingrong Shena and Liang Fang*a, a College of Physics, Optoelectronics and Energy and Jiangsu Key Laboratory of Thin Films, Soochow University, Suzhou, 215006, People’s Republic of China b Institute of Functional Nano& Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-based Functional Materials and Devices, and Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215006, People’s Republic of China c School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore * Author to whom correspondence should be addressed. e-mail: zhkang@suda.edu.cn; lfang@suda.edu.cn Summary: There are 7 pages including 3 figures 1 Preparation of io-BiVO4/CQDs photoelectrode Fluorine-doped SnO2 (FTO) coated glass substrates were cleaned by immersing in acetone, ethanol and deionized water for 0.5 h each with sonication. Mono-dispersed polystyrene (PS) spheres (SuZhou Nanomicro Tech.) were self-assembled onto the FTO-coated glass substrates. Subsequently, the self-assembled PS opals were infiltrated with BiVO4 precursor to form io-BiVO4 using dip-coating sol-gel technique. The precursor is produced as follows: 0.005 mol of Bi(NO3)3, 0.005 mol of NH4VO3 and 0.01 mol of citric acid were dissolved in 15 mL of 23.3% HNO3 aqueous solution. Then 0.08 g of polyvinyl alcohol and 0.25 mL of acetic acid were added into 1 mL of this blue color solution. The resulting mixture was stirred to promote the dissolution and reaction until the mixture turned into a transparent solution. The opal template was then dipped into the precursor sol for 5 min. The samples were dried for 1 h in an oven at 80 °C to transform the sol into a gel. The infiltration and drying processes were repeated 3 times to ensure that the voids in the template were completely filled. The sample was finally annealed in air at 350 °C for 2 h to crystallize monoclinic BiVO4. The CQDs were prepared using electrochemical etching method. Two graphite rods (99.99%, Alfa Aesar Co. Ltd., 13 cm in length and 0.6 cm in diameter), acting as the anode and cathode, were inserted in a beaker containing 800 ml ultrapure water (18.4 MΩ cm) at a distance of 7.5 cm. An dc bias of 60 V was applied between the two electrodes for eight days combined with magnetic stirring. Subsequently, the black solution was filtered and centrifuged at 22000 rpm for 20 minutes to eliminate the large graphite particles. Finally, a 0.1mg/ml CQDs solution was obtained using rotary evaporation at 70 °C. The CQDs coated io-BiVO4 (io-BiVO4/CQDs) were fabricated using a constant-voltage electrodeposition method, which was performed in a two-electrode mode at an applied potential of 1.0 V for 5 min with a coiled Pt wire as the counter electrode. Characterization The morphologies of the samples were characterized using a JEOL JSM-6700F 2 field-emission scanning electron microscope (FESEM). Transmission electron microscopy (TEM) images were obtained using a Tecnai G2F20 electron microscope at an acceleration voltage of 200 kV. The X-ray diffraction (XRD) patterns of the samples were measured using a Shimadzu thin film diffractometer equipped with Cu Kα radiation (λ=1.540598Å). The ultraviolet-visible (UV-vis) absorption spectra of the samples were obtained using a Shimadzu 3600 UV-Vis-NIR spectrophotometer. Fourier transform infrared (FTIR) spectra were recorded on a Varian 3600 FTIR spectrometer. Photoluminescence spectra were measured by Jobin Yvon Time-correlated Single Photon Counting System (Horibfm-2015). Photoelectrochemical measurements Photocurrent density and electrochemical impedance spectroscopy (EIS) measurements were performed using electrochemical analyzer (CHI-660D, Shanghai Chenhua Instrument Co. Ltd.) in a standard three-electrode configuration under 300W Xe-lamp illumination. The different BiVO4 photoelectrodes acted as the photoanode, Pt wire as the counter electrode, and Ag/AgCl as the reference electrode. The electrolyte was 0.1 M Na2SO4 aqueous solution (pH=7). Photocurrents were measured under applied potentials of 0 to 1.35 V vs. RHE (reverseble hydrogen electrode) by using Nernst equation:1 ERHE =EAg/AgCl + E0Ag/AgCl + 0.059 pH (1) where ERHE is the converted potential versus RHE. EAg/AgCl is the external potential measured against the Ag/AgCl reference electrode. E0Ag/AgCl is the standard potential of Ag/AgCl at 25 oC (0.1976 V). EIS spectrum was obtained by applying an open-circuit voltage (about 0.65 V) in the frequency range of 0.01 Hz to 10 MHz with oscillation amplitude of 10 mV. Before performing EIS measurement, the measurement cell was discharged to a designated potential, then kept under open-circuit condition for 1 h to ensure the equilibrium of cell. PEC water splitting was carried out in an Ar gas environment with a flow rate of 10 mL min-1. The amounts of produced gases were determined with gas chromatography (Shimadzu; MS-5A column, Ar carrier, TCD). The light source was a 3 300 W Xe-lamp. The working electrode was irradiated with light from BiVO4 side. The electrolytes in both compartments were bubbled with N2 or Ar before measurements to remove dissolved O2. In order to utilize the slow light effect of the photonic crystal, the appropriate size of the PS spheres has to be determined to match the stop-band of io-BiVO4. Considering the application of this photoelectrode in the subsequend water treatment experiments, the stop-band of io-BiVO4 can be calculated according to the modified Bragg's law: 2 2 2 2 D nBiVO f nwater 1 f sin 2 4 3 (2) where λ is the wavelength derived from the stop-band, D is the pore size of the inverse opal, nBiVO4 (2.45) and nwater (1.33) are the refractive indexes of BiVO4 and water, respectively. f is the BiVO4 phase volume percentage, which is generally taken as 0.26, and θ is the incident angle of light. For normal incidence, θ=0. According to the equation 2 and considering the shrinkage (~ 40%) of the pores during the removal of PS template by sintering,2,3 the template spheres with diameter of 260 nm were selected to fabricate the io-BiVO4. Since the Bragg diffraction peak is centered at 415 nm, the position of the slow light propagation at the red edge of the photonic band gap is in the vicinity of 450 nm.2,4 Therefore, the red edge of the stop-band is overlapped with the optical absorption edge of our BiVO4 samples (Figure S1), indicating that the light absorption of BiVO4 can propagate with strongly reduced group velocity, and increase the effective path length of light. 4 Figure S1 Diffused reflectance UV-Vis spectra of the io-BiVO4 sample. The shadow region is the optical absorption region of BiVO4. The location of slow light at the red-edge of stop-band for the io-BiVO4 sample is shown as hollow circle. Figure S2 The XRD patterns of the different samples. 5 Figure S3 Chronoamperometry measurements of the io-BiVO4/CQD samples with different CQDs electrodeposition times Figure S4 The time-dependent photocurrent profile obtained for all the samples during the gas collection cycles. 6 REFERENCE 1 S. Hoang, S. W. Guo, N. T. Hahn, A. J. Bard and C. B. Mullins, Nano Lett. 12, 26 (2012). 2 Y. Lu, H. T. Yu, S. Chen, X. Quan, and H. M. Zhao, Environ. Sci. Technol. 46, 1724 (2012). 3 X. Q. Chen, J. H. Ye, S. X. Ouyang, T. Kako, Z. S. Li, and Z. G. Zou, ACS Nano 5, 4310 (2011). 4 J. I. L. Chen, G. V. Freymann, S. Y. Choi, V. Kitaev, and G. A. Ozin, Adv. Mater. 18, 1915 (2006). 7