Half-reactions and Electrochemical Cells
advertisement

Tutorial schedule
(3:30 – 4:50 PM)
• No. 1 (Chapter 7: Chemical Equilibrium)
January 31 at Biology building, room 113
February 1 at Dillion Hall, room 254
• No. 2 (Chapter 22: The rates of chemical
reactions)
March 6 at Biology building, room 113
March 7 at Dillion Hall, room 254
• No. 3 (Chapter 24: Molecular Reaction
Dynamics)
March 27 at Biology building, room 113
March 28 at Dillion Hall, room 361
Extended Debye-Hückel law
•
A | z z | I 1 / 2
log( )
1 BI 1 / 2
B is an adjustable empirical
parameter. It is different for each
electrolyte.
Calculating parameter B
Example : The mean activity coefficient of NaCl in a diluted aqueous solution at
25oC is 0.907 (at 10.0 mmol kg-1). Estimate the value of B in the extended
Debye-Huckel law.
Solution: First calculate the ionic strength
I = ½[12*0.01 + (-1)2*0.01] = 0.01
A | z z | I 1 / 2
From equation log( )
1 BI 1 / 2
log(0.907) = - (0.509|1*(-1)|*0.011/2)/(1+ B*0.011/2)
B = - 1.67
Half-reactions and electrodes
Two types of electrochemical cells:
1. Galvanic cell: is an electrochemical cell which
produces electricity as a result of the spontaneous
reactions occurring inside it.
2. Electrolytic cell: is an electrochemical cell in which
a non-spontaneous reaction is driven by an external
source of current.
• Other important concepts include:
Oxidation: the removal of electrons from a species.
Reduction: the addition of electrons to a species.
Redox reaction: a reaction in which there is a transfer of electrons
from one species to another.
Reducing agent: an electron donor in a redox reaction.
Oxidizing agent: an electron acceptor in a redox reaction.
• Two type of electrodes:
Anode: the electrode at which oxidation occurs.
Cathode: the electrode at which reduction occurs
Typical Electrodes
Electrochemical cells
• Liquid junction potential: due to the
difference in the concentrations of
electrolytes.
• The right-hand side electrochemical
cell is often expressed as follows:
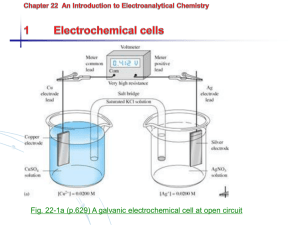
Zn(s)|ZnSO4(aq)||CuSO4(aq)|Cu(s)
• The cathode reaction (copper ions
being reduced to copper metal) is
shown on the right. The double bar (||)
represents the salt bridge that separates
the two beakers, and the anode
reaction is shown on the left: zinc
metal is oxidized into zinc ions
In the above cell, we can trace the movement of charge.
–
–
–
–
Electrons are produced at the anode as the zinc is oxidized
The electrons flow though a wire, which we can use for electrical energy
The electrons move to the cathode, where copper ions are reduced.
The right side beaker builds up negative charge. Cl- ions flow from the salt bridge
into the zinc solution and K+ ions flow into the copper solution to keep charge
balanced.
To write the half reaction for the above cell,
Right-hand electrode: Cu2+(aq) + 2e- → Cu(s)
Left-hand electrode: Zn2+(aq) + 2e- → Zn(s)
The overall cell reaction can be obtained by subtracting
left-hand reaction from the right-hand reaction:
Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
Expressing a reaction in terms of
half-reactions
Example : Express the formation of H2O from H2 and O2 in
acidic solution as the difference of two reduction halfreactions.
(in class discussion)
Redox couple: the reduced and oxidized species in a halfreaction such as Cu2+/Cu, Zn2+/Zn….
Ox + v e- → Red
The quotient is defined as: Q = aRed/aOx
Example: Write the half-reaction and the reaction quotient
for a chlorine gas electrode.
(in class discussion)
Varieties of cells
Notation of an electrochemical cell
• Phase boundaries are
denoted by a vertical
bar.
• A double vertical line,
||, denotes the
interface that the
junction potential has
been eliminated.
• Start from the anode.
A general format:
Solid | gas phase | aqueous phase || aqueous phase | gas phase | solid
Concentration Cells
•
M | M+(aq, L) || M+(aq, R) | M
• Cell reaction: M+(aq, R) → M+(aq, L)
r G RT
RT
E
ln Q E
ln Q
vF
vF
vF
since
ΔrGθ = 0 (why?)
E
RT bL
ln
vF bR
Cell Potential
•
Cell potential: the potential difference between two electrodes of a
galvanic cell (measured in volts V).
•
Maximum electrical work : we,max = ΔG
•
Electromotive force, E,
•
Relationship between E and ΔrG:
ΔrG = -νFE
where ν is the number of electrons that are exchanged during the
balanced redox reaction and F is the Faraday constant, F = eNA.
•
At standard conditions, this equation can be written as
ΔrGθ = -νFEθ
The cell emf
• The emf of a cell can be calculated by the difference of the potentials of
the two electrodes, Ecell = Eright - Eleft
• The potential of an electrode can be calculated from its standard
potential. For example
Fe+3(aq) + e- → Fe+2(aq)
RT bFe2
E E
ln
vF
bFe3
Cu+2(aq) + 2e- → Cu(s)
E E
RT aCu
ln
vF
bCu 2
• Consider: Ag(s)|Ag+(aq) || Cl-(aq) |AgCl(s)| Ag(s)
Ecell = E(AgCl/Ag, Cl-) – E(Ag+/Ag) = Eθ - ??
• In the lead storage battery (used in
automobiles),
Pb | PbSO4 | H2SO4 | PbSO4|PbO2 | Pb
would the voltage change if you changed the
concentration of H2SO4? (yes/no)
• Answer ...
• Yes, because
• The net cell reaction is
Pb + PbO2 + 2HSO4- + 2H+ → 2 PbSO4 + 2 H2O
• The Nernst equation
E = E° - (0.0594/2)log{1/{[HSO4-]2[H+]2}}.
Previous two examples involve the same number of electron transfer at the
cathode and anode, how to calculate emf if the number of electrons
transferred at the two electrodes are different?
Example: What is the emf for the cell : Mn(s)|Mn+2||Fe+3|Fe+2|Pt(s)
Solution: The two reduction half reactions
Right: Fe+3(aq) + e- → Fe+2(aq)
Left:
Mn+2(aq) + 2e- → Mn(s)
It shows that the above two half reactions have different number of electrons
being transferred!
The cell reaction is obtained via 2*R – L,
2Fe+3(aq) + Mn(s) → 2Fe+2(aq) + Mn+2(aq)
should the standard cell potential be calculated as 2*Eө(R) - Eө(L) ?
Answer: NO! it is still calculated with Ecell = Eright - Eleft
Consider:
ΔrGθ = 2ΔrGθ (R) - ΔrGθ(L)
2FE θ = 2(1*F* E θ (R) – 2*F* E θ (L)
it leads to
Eөcell = Eө(R) - Eө(L)
= 0.769 - (- 1.182) = 1.951 V
Standard Cell emf
•
•
Eθcell = Eθ (right) – Eθ(Left)
Calculating equilibrium constant from the standard emf :
Evaluate the solubility constant of silver chloride, AgCl, from cell potential
data at 298.15K.
Solution:
AgCl(s) → Ag+(aq) + Cl-(aq)
Establish the electrode combination:
Right: AgCl + e- → Ag(s) + Cl-(aq) Eθ = 0.22V
Left:
Ag+(aq) + e- → Ag(s)
Eθ = 0.80V
The standard cell emf is : Eθ (right) – Eθ(Left) = - 0.58V
vFE
0.58
ln K
RT
0.0257
K = 1.6x10-10
•
The above example demonstrates the usefulness of using two half reactions
to represent a non redox process. What would be the two half reactions for
the autoprotolysis of H2O?
The measurement of standard
potentials
• The potential of standard hydrogen electrode:
Pt(s)|H2(g)|H+(aq)
is defined as 0 at all temperatures.
• The standard potential of other electrodes can be obtained by
constructing an electrochemical cell, in which hydrogen electrode is
employed as the left-hand electrode (i.e. anode)
• Example: the standard potential of the AgCl/Ag couple is the
standard emf of the following cell:
Pt(s)|H2(g)|H+(aq), Cl-(aq)|AgCl(s)|Ag(s)
or
Pt(s)|H2(g)|H+(aq) || Cl-(aq)|AgCl(s)|Ag(s)
with the cell reaction is: ½ H2(g) + AgCl(s) → H+(aq) + Cl-(aq) + Ag(s)