Beer's Law Lab Analysis of Dye in Gatorade
advertisement
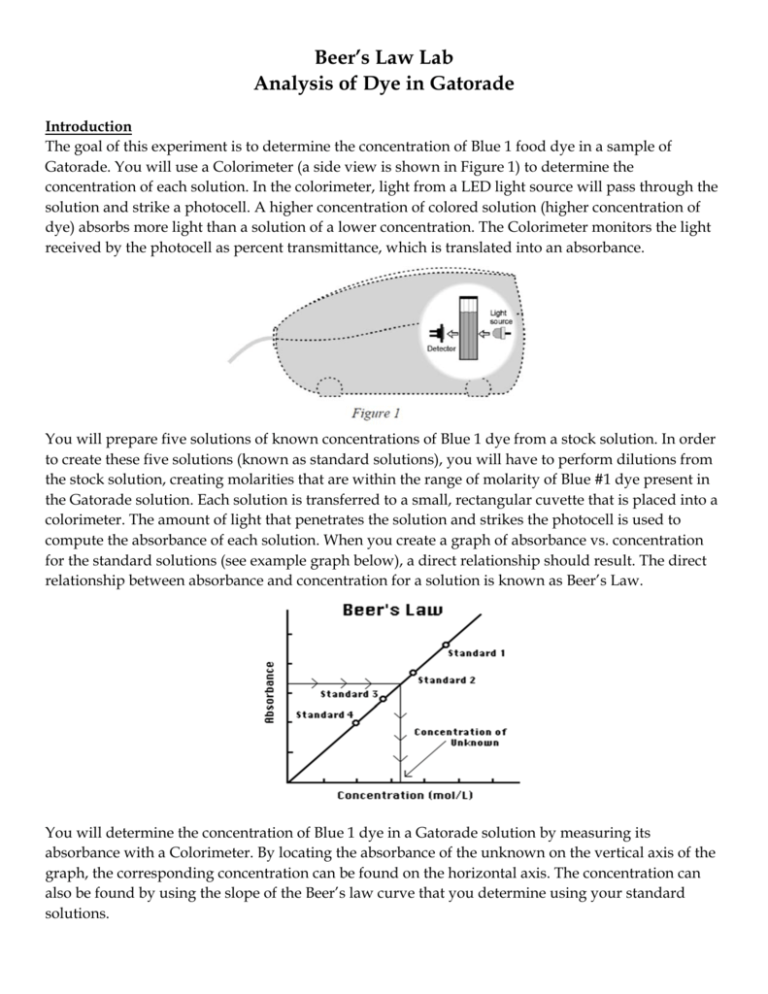
Beer’s Law Lab Analysis of Dye in Gatorade Introduction The goal of this experiment is to determine the concentration of Blue 1 food dye in a sample of Gatorade. You will use a Colorimeter (a side view is shown in Figure 1) to determine the concentration of each solution. In the colorimeter, light from a LED light source will pass through the solution and strike a photocell. A higher concentration of colored solution (higher concentration of dye) absorbs more light than a solution of a lower concentration. The Colorimeter monitors the light received by the photocell as percent transmittance, which is translated into an absorbance. You will prepare five solutions of known concentrations of Blue 1 dye from a stock solution. In order to create these five solutions (known as standard solutions), you will have to perform dilutions from the stock solution, creating molarities that are within the range of molarity of Blue #1 dye present in the Gatorade solution. Each solution is transferred to a small, rectangular cuvette that is placed into a colorimeter. The amount of light that penetrates the solution and strikes the photocell is used to compute the absorbance of each solution. When you create a graph of absorbance vs. concentration for the standard solutions (see example graph below), a direct relationship should result. The direct relationship between absorbance and concentration for a solution is known as Beer’s Law. You will determine the concentration of Blue 1 dye in a Gatorade solution by measuring its absorbance with a Colorimeter. By locating the absorbance of the unknown on the vertical axis of the graph, the corresponding concentration can be found on the horizontal axis. The concentration can also be found by using the slope of the Beer’s law curve that you determine using your standard solutions. FD&C Dyes Food colors have been used for centuries because we have come to expect that our food will look a certain way. We expect that orange flavored drinks will be orange, and for our lab, that blueberrypomegranate Gatorade will be blue. Lime and orange sherbets would be nearly indistinguishable based on appearance if not for the green and orange dyes. In addition, coloring agents add a festive appearance to foods. M&M candies would certainly be less appealing if they were all colored gray. How many of you have ordered a snow cone or other item based on color? Historically, coloring agents have also been added to foods to cover up inferior quality foods. At the beginning of the 20th century, when there was no regulation of color additives in this country, coloring agents were added to foods to mask spoiled foods, and some coloring agents marketed for inclusion in foods were poisonous. Since the passage of the Federal Food, Drug, and Cosmetic (FD&C) Act of 1938, color additive in the U.S. have been the responsibility of the Food and Drug Administration (FDA). Dyes are still used to cover inferior products, but now the dyes must be approved (and the food must also meet basic quality inspections). At present, there are seven color additives that are certified for food use: Blue 1, Blue 2, Yellow 5, Yellow 6, Green 3, Red 3, and Red 40. Most are organic molecules similar to brilliant blue (FD&C Blue 1) that will be studied in this experiment. Its structure is shown below. FD&C Blue 1 Dye – C37H34N2Na2O9S3 – 792.85 g/mol Experiment 1. Obtain 15 mL of FD&C Blue 1 stock solution from the back of the room. 2. Obtain ~150 mL of distilled water in a beaker. 3. Setup the LabQuest for the Colorimeter a. Plug the Colorimeter into Ch. 1 on the LabQuest. Set it to 630 nm wavelength. b. Fill a blank cuvette ¾ full with distilled water. Wipe it dry and place it in the Colorimeter with the clear side facing the arrow in the Colorimeter. Close the lid. c. Press the Cal button and wait for the red light to finish flashing. d. Remove the cuvette and empty the water. This is the only time calibration is needed. 4. Prepare the first of five standard solutions. Use a pipet to obtain 1 mL of stock solution and place it in a 25 mL graduated cylinder. Add distilled water to a total volume of 25 mL. 5. Determine the molarity of the diluted solution and record it in the Data Table. 6. Pour the solution into a mixing cup and swirl the solution to ensure that it mixes completely. 7. Use a pipet to fill your cuvette ¾ with the solution. Place in the Colorimeter and close the lid. 8. Record the absorbance in the Data Table. 9. Repeat steps 4-8 for additional standard solutions. Use 2, 3, 4, and 5 mL of stock solution and dilute each to 25 mL. 10. Obtain 5 mL of Blue Gatorade in a 10 mL graduated cylinder. Add 5 mL of distilled water. 11. Pour the solution into a mixing cup and swirl the solution to ensure that it mixes completely. 12. Fill the cuvette ¾ full with the solution, place in the Colorimeter, and record the absorbance. Data/Calculations Calculate the molarity of the original stock solution, which was made with 0.0565 g of Blue 1 in 1400mL of solution. Show all work and units!! Calculate the molarity of each diluted standard solution and fill in the data table. Show at least one sample calculation for the concentration. (Hint: M1V1 = M2V2) Data Table Standard Volume of stock solution (mL) Total solution volume after dilution (mL) A 1 25 B 2 25 C 3 25 D 4 25 E 5 25 Concentration of diluted sample (M) Absorbance Gatorade Create a graph of absorbance vs. concentration for the 5 standard solutions. Draw a best-fit line. Determine the line equation for the line _____________________________________ Analysis Questions – SHOW ALL WORK!!! 1. Use the graph/best fit-line to determine the concentration of Blue 1 in the Gatorade solution. 2. Consider the concentration from #1. The solution was diluted 1:2, so this represents ½ of the actual molarity. Determine the actual molarity of Blue 1 in the Gatorade solution. 3. List 3 sources of error that could have occurred in this lab. 4. Use the internet to research FD&C Blue 1 dye. Write about any side effects, both positive and negative, of consuming this dye. 5. Consider the following data of absorbance vs. concentration for a solution containing Cu2+ ion. Create graph of absorbance vs. concentration and answer the following questions. Sample Concentration (M) 1 0.0016 2 0.0048 3 0.0080 4 0.0112 5 0.0128 Unknown ? Absorbance 0.069 0.183 0.334 0.457 0.557 0.239 a. Determine the concentration of unknown. Show all work. b. Determine the absorbance of a solution with a concentration of 0.0100M. c. Why does this curve help us determine the answers to questions a & b?











