ME 354 Tutorial #2 – Availability
advertisement

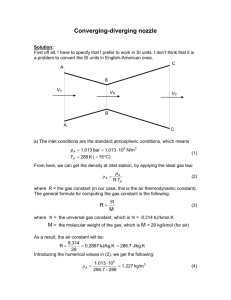
ME 354 Tutorial 2B – Exergy – Control Volume Analysis Winter 2001 In the boiler of a typical power plant, H2O flows inside the tubes lining the walls of the combustion chamber. Consider a case in which the H20 is brought from 0.8 Mpa & 150C to 250C at essentially constant pressure while the combustion gases passing over the tubes cool from 1100C to 500C at essentially constant pressure. The gases can be modeled as nitrogen (N2). There is no significant heat transfer from the combustion chamber to the surroundings. Find: a) the mass flow rate of combustion gases per kg of H20 flowing inside the tubes b) the rate of exergy destruction per kg of H20 flow Step 1: Draw a diagram to represent the system (show control volume of interest) The first control volume encloses the H2O pipes as shown below The second control volume encloses all the N2 in the combustion chamber. Step 2: Prepare a property table H2O N2 State 1 2 3 4 T [K] 523.15 343.15 1373.15 773.15 Property P [MPa] h [kJ/kg] s [kJ/kg*K] 0.8 0.8 P3 P3 Step 3: State your assumptions Assumptions: 1) SSSF (steady state/steady flow) 2) Combustion gases modeled as N2 – Ideal Gas 3) No heat transfer from system to surroundings 4) ke, pe 0 5) Non-constant specific heats for N2 Step 4: Calculations (usually start by writing First and Second Laws) Part a) Writing the First Law for the control volume around the H20 tubes gives Eq1 dEcv m1 (e Pv)1 m 2 (e Pv) 2 Q W dt (Eq1) Expanding the energy terms (e = u + ke + pe), realizing that there is no shaft work into the pipes, and applying the SSSF assumption dE ( cv 0, m1 m 2 m H2O), Eq1 can be written as Eq2. dt . . 0 m H 2O (u ke pe Pv)1 (u ke pe Pv) 2 Q12 (Eq2) Using the assumption that ke and pe are approximately zero, and the fact the property enthalpy, h, is defined as u + Pv, Eq2 can re-written as Eq3. . . Q 12 m H 2O h2 h1 (Eq3) Applying the above analysis to the N2 control volume (noting that the heat transfer is now out of the system) yields Eq4. - Q 34 m N 2 h4 h3 (Eq4) From examination of the control volume diagrams we can see that the heat transfer from the N2 is equal to the heat transfer into the H20. Eq5 expresses this relation. (Eq5) Q 12 Q 34 Relating Eq3 & Eq4 through Eq5 gives m H 2O h2 h1 m N 2 h3 h4 (Eq6) We need to find the ratio of the mass flow rate of combustion gases, m N 2 , to mass flow rate of H20, m H 2 O . Rearranging Eq6 into the form of Eq7. mN2 m H 20 h2 h1 h3 h4 (Eq7) To determine the enthalpy of N2 at state 3 and 4, we can use Table A-18 (since there is a 600 temperature difference between state 3 and 4, the constant specific heat assumption could lead to considerable error. This is why we are not using the relation h 4 - h3 = cp(T4-T3)). Table A-18 gives enthalpy on a kmol basis. To convert to a kg basis, we can use Table A-1, which gives the molar mass of N2 as 28.02 kg/kmol. From Table A-18, interpolating between T=1360K and T=1380K for T3= 1373.15K (1100C) _ _ 1360 1373.15 42227 h 3 h 3 42679.4 kJ/kmol 1360 1380 42227 42915 h3 = (42679.4 kJ/kmol)/(28.02 kg/kmol) = 1523.2 kJ/kg From Table A-18, interpolating between T=770K and T=780K for T4= 773.15K (500C) _ _ 770 773.15 22772 h 4 h 4 22870.6 kJ/kmol 770 780 22772 23085 h4 = (22870.6 kJ/kmol)/(28.02 kg/kmol) = 816.2 kJ/kg To determine the enthalpy of H20 at state 1 and 2, we can use the steam tables. At state 1, the temperature of the H20 is 150C @ 0.8MPa. Looking first in Table A-5 at 0.8MPa we find that the corresponding saturated temperature is 170.43C. Since T1 < 170.43C, the H20 at state 1 is subcooled (compressed liquid). We can approximate the enthalpy at state 1 using the enthalpy of saturated liquid at 150C (see p73 of text for explanation). From Table A-4 @ 150C h1 = hf =632.20 kJ/kg At state 2, the temperature of the H20 is 250C @ 0.8MPa. Looking first in Table A-5 at 0.8MPa we find that the corresponding saturated temperature is 170.43C. Since T2 > 170.43C, the H20 at state 2 is superheated. We can use Table A-6 @ 0.8MPa to determine the enthalpy of state 2. From Table A-6 @ 250C, 0.8 MPa h2 = 2950.0 kJ/kg We can now substitute these values into Eq7 to solve for the mass flow ratios. mN2 m H 20 h2 h1 2950.0 632.20 = 3.28 h3 h4 1523.2 816.2 Answer a) Part b) To find the rate of exergy destruction, I , we can make use of the generalized exergy equation (Week 3: Lecture 3) shown in Eq8 applied to the entire system – see above diagram for new control volume boundary. dCV dV T T P0 CV W m Q1 0 W m Q1 0 I dt dt TTER IN TTER OUT (Eq8) With our new control volume boundary, there is no work or heat transfer out of the system. Also, with the steady state assumption, the time derivatives in Eq8 go to zero. Eq8 can be reduced to Eq9. I m m IN OUT (Eq9) The flow exergy terms in Eq9 can be expanded in terms of the individual flow exergy terms of the system as shown in Eq10. I m H 2 O 1 mN 2 3 m H 2 O 2 mN 2 4 (Eq10) The question has asked for the exergy destruction per kg of H2O flow. We can obtain an expression for this by dividing Eq10 through by m H 2 O to obtain Eq11. I 1 2 m H 2O mN 2 3 4 (Eq11) m H 2O We determined mN 2 in part a, so the question has been reduced to finding the m H 2O differences in the flow exergies. Referring to Week 3: Lecture 3, the flow exergy can be determined from Eq12. h h0 T0 s s0 g z z 1 2 v v0 2 2 0 (Eq12) The difference in flow exergy can now be expressed as shown in Eq13. 1 2 h1 h2 T0 s1 s 2 1 v1 2 v g z 2 2 2 1 z2 (Eq13) Using the assumption that ke & pe 0, Eq13 reduces to Eq14. 1 2 h1 h2 T0 s1 s2 Similarly, applying the same steps for 3 – 4 we obtain Eq15. (Eq14) 3 4 h3 h4 T0 s3 s4 (Eq15) We determined the enthalpies in part a) we now must similarly determine the entropies. From Table A-18, interpolating between T=1360K and T=1380K for T3= 1373.15K (1100C) _ _ 1360 1373.15 238.376 s 3 s 3 238.706 kJ/kmol*K 1360 1380 238.376 238.878 s3 = (238.706 kJ/kmol*K)/(28.02 kg/kmol) = 8.519 kJ/kg*K From Table A-18, interpolating between T=770K and T=780K for T4= 773.15K (500C) _ _ 770 773.15 219.709 s 4 s 4 219.836 kJ/kmol*K 770 780 219.709 220.113 s4 = (22870.6 kJ/kmol)/(28.02 kg/kmol) = 7.846 kJ/kg*K From Table A-4 @ 150C s1 = sf =1.8418 kJ/kg*K From Table A-6 @ 250C, 0.8 MPa s2 = 7.0384 kJ/kg*K Substituting in these values in to Eq13 and Eq14. 1 2 632.20 2950 25 273.151.8418 7.0384 =-768.43 kJ/kg (Eq13) 3 4 1523.2 816.2 25 273.158.519 7.846=506.36 kJ/kg (Eq14) Substituting Eq13 & Eq14 into Eq11, I 768.43 3.28506.36 =892.43 kJ/kgH2O m H 2O Step 5: Concluding Statement & Remarks Answer b) The mass flow rate of combustion gases per kg of H20 flowing inside the tubes was found to be 3.28. The rate of exergy destruction per kg of H 20 flow was found to be 892.43 kJ/kgH20.








![M&M Lab Report Template [11/1/2013]](http://s3.studylib.net/store/data/007173364_1-88fa2a4b33d860a6d8d06b9423fde5c0-300x300.png)
