Stotz
advertisement

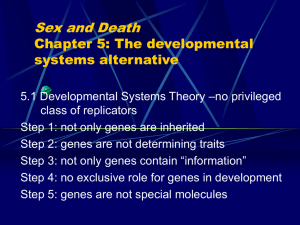
Developmental Niche Construction: an integrative causal factor in evolution (Commentary on Pigliucci and Kaplan’s Making Sense of Evolution) Karola Stotz 1. Introduction Pigliucci and Kaplan’s rather critical evaluation of contemporary evolutionary biology starts with making a very useful and necessary distinction three levels of analysis of evolutionary processes, the individual, population and ensemble level. While the first two contain the actual physical, causal processes that lead to and make possible evolution (by natural selection), the latter measures statistical distributions of outcomes. The authors acknowledge and attempt to conceptually integrate several new developments which play an important role to understand causal processes at the first two levels that strengthen the active role (populations of) organisms and their development play in evolution. These accounts are Developmental Systems Theory, which includes and takes serious the idea of Extended Inheritance, a variety of accounts that run under the heading of Evo-Devo, Eco-Devo and Devo-Evo, Phenotypic Plasticity including mechanism such as genetic accommodation and assimilation, and Niche Construction. This review extends the authors’ treatment of what kind of questions evolutionary biology is concerned with and which kind of answers would provide their explanations. Evolutonary biology should explain “ the origin, spread, and maintenance of phenotypic traits, as well as the developmental pathways that reliably (re)produce them” (Pigliucci and Kaplan 2007, 112). I want to draw attention to the developmental niche and its active construction through (populations of) organisms and their parental generation, a process which has been first been described by Meredith West and Andrew King in 1987, a year earlier than the first publication about the process of niche construction by Odling-Smee (West and King 1987; Odling-Smee 1988). While niche construction has since then almost become a household name in evolutionary theory, not so the idea of the developmental niche. The importance of the mechanism of ontogenetic niche construction lies in its integrative power: it combines ideas of the active organism altering its environment (niche construction), developmental systems theory and extended (non- or extra-genetic) inheritance, evo-eco-devo and phenotypic plasticity. Because of the role it plays in explanations of all of the above questions its integration into mainstream evolutionary theory will be necessary for the continued refinement of this evolving field. The process of developmental niche construction contributes to explanation of: a) the origin of a trait by introducing new epigenetic resources for variation and innovation beyond mutation and recombination and describing how developmental processes situated in their ecological niche can produce novel phenotypes; b) the spread of a trait by showing in detail how organisms or their parental generation co-construct a selective environment; and c) the maintenance of a trait through processes of transgenerational stability of variation that extend the inheritance through the transmission of genetic material with the reliable availability of necessary developmental resources through multiple mechanisms of reproduction or transmission. My central claim is that any scientific understanding of the nature of living things, including its evolution, depends crucially on our understanding of the most basic of biological processes that brought them about: development. For the longest time this was not generally accepted by the founders and proponents of the Modern Synthesis for three reasons: a) The misconstruction of development as the mere unfolding or maturation of the organism out of its genetic ‘blueprint’ or’ program’, instead of its emergence as qualitative novelty out of an more or less undifferentiated and unformed mass; b) The reduction of inheritance and transgenerational stability of traits to the transmission of genetic ‘information’; and c) The neglect of the problem of evolutionary novelty, the socalled ‘arrival of the fittest’. Taken together, it has not taken seriously the physical, causal processes that make possible the creation of adaptation through natural selection, namely reliably reproducing developmental systems. In other words, evolutionary biology has hitherto missed to answer the very possibility of evolution through the variability, adaptability and evolvability of phenotypes. A new evolutionary synthesis needs to include: 1) A realistic view of gene action, activation and regulation, that is of pivotal importance to wider theory of evolution since it stresses the fact of the underdetermination of phenotypic traits through genetic information and hence the necessity for extended systems of inheritance to supplement this information. 2) A new understanding of the nature of inheritance, which includes maternal effects on gene expression, epigenetic factors such as genetic imprinting, and behavioral, ecological, social, cultural and symbolic inheritance systems. 3) the nature of stably reproduced, adaptive developmental systems that are sustained through the process of ontogenetic niche construction. 2. The Modern Consensus of Evolution, Heredity and Development The turn of the last century saw two important scientific developments that are important in order to understand our modern understanding of development, evolution and heredity. Most importantly, its saw the rediscovery of Mendel and the emergence of classical transmission genetics which ultimately led to its split from embryology and its integration with Darwin’s theory of evolution by natural selection into Neo-Darwinism and then the Modern Synthesis under the exclusion of embryology. All the while the rise of developmental mechanics with its new methodology of manipulating animals in controlled laboratory settings brought the discipline of embryology, now called developmental biology, from the sea shore to the indoors. This constrained the choice of organism, which “must be selected for the inability of their development to be influenced by specific environmental cues”. In other words, “the influence of … environmental sources of phenotypic diversity were progressively eliminated under the physiological context of embryology” (Gilbert 2003, 88f). While this physiological tradition favored the whole organism at the expense of the environment, transmission and population genetics and the later emerging molecular genetics focused on genes at the expense of the organism. Both research traditions discounted and dispensed with both the organism and the environment understood as both the co-constructed niche of the organism and the internal cellular environment of the gene and their expression. The ‘century of the gene’ (Keller 2000) has brought about a new and more sophisticated preformationism that replaced the older ‘homunculus’ as the preformed organism by the ‘information’ for making an organism encoded in the genome. This new conception is less a preformationism rather than a kind of ‘animistic’ predeterminism, where genes ‘program’ and therefore predetermine, less than perform, outcomes. True to the spirit of today’s interactionism the mainstream ‘modern consensus’ can be “standardly construed as the epigenesis of something preformed in the DNA” (Robert 2004, 34). It rest, quite problematically, on an unscientific conception of gene and gene action. In its place I want to promote ‘molecular epigenesis’, a bold thesis about the contingency and epigenetic regulation of gene expression. It proposes three classes of phenomena, sequence ‘activation’, ‘selection’ and ‘creation’, replacing the misleading metaphor of ‘gene action’. They more accurately describe what happens during the expression of genes through transcriptional, pre-, co-, and post-transcriptional processes of DNA coding sequences. Molecular Epigenesis contains three sub-theses: 1) Thesis of distributed causal specificity: Other molecular resources share the causal role of ‘genes’: the ‘causal specificity’ for the linear sequence of any gene product is distributed between the coding sequence, cis-acting sequences, trans-acting factors, environmental signals, and the contingent history of the cell (the cellular code). 2) Thesis of genetic underdeterminism: These multiple and overlapping processing and targeting mechanisms amplify the repertoire of RNA and protein products specified through the eukaryotic genome, expanding the possibilities specified by the literal code of DNA. 3) Thesis of regulated recruitment, combinatorial control, and systemic organization: These mechanisms of gene expression change the focus of postgenomic research from single molecules and their molecular, biochemical and intrinsic function to their cellular, constituent, component or contextual function due to their recruitment and organization in complex cellular networks. In other words, all agents involved in the regulation of gene expression, including DNA, must interact with other agents to achieve full specificity, which is imposed by regulated recruitment and combinatorial control. I conclude from these three main theses that organisms necessarily inherit stable developmental resources that ensure the reliable differential expression of modular genetic resources. 3. Epigenetic regulation of gene expression Genetic activity is involved in all biological processes, but so are non-genetic factors. Explanations listing only interacting genes are vacuous statements. Postgenomic biology has brought with it a new conception from the active gene to the reactive genome that is activated and regulated by cellular processes that include signals from the internal and external environment (Stotz forthcoming). This is not the place to report the details now available on the mind-numbing complexities of the expression of genes during development, instead a few detail should suffice. The last decade of genome-sequencing has revealed the paradox that the complexity of organism is not related to its number of genes. Instead, it seems to be related to the complexity of the expression of this limited number of coding sequences. In other words, what is of particular importance during development is not the existence of some genes but their differential time- and tissuedependent expression. In the last two decades development has become equated with differential gene expression, but what is often forgotten in this definition is the complex network of other molecules (such as proteins and metabolites), cellular structures, 3dimentional cellular assemblages and other higher-level structures that control or are otherwise involved not only in this differential expression of genes but in a wide range of other developmental processes decoupled from the direct influence of DNA sequences. In eukaryotes DNA is part of a densely packed chromatin structure, which allows it to fit neatly into the nucleus, but which is also a major mechanism to control gene expression. The DNA’s weak chemical bond to the histone complex to which it is tightly wrapped to form nucleosomes (like beads on a string) needs to be broken down in order to free the DNA molecule to undergo new bonds with transcription factors. Hence the default position of DNA in eukaryotes is no expression unless expression is activated. Several large complexes of transcription factors and several other accessory proteins such as chromatin remodeling factors are needed in order to proceed with the transcription of a stretch of DNA. Most of these factors are themselves gene products. What is often not mentioned, however, is the need for environmental inducers for all transcriptional processes. It generally holds for all eukaryotes that “in the absence of their respective inducing signal, transcriptional regulators tend not to be found in the nucleus with (in the case of activators) their activating regions free to work. Rather, activating regions are masked … or… the regulators are maintained outside of the nucleus, until the inducing signal is detected” (Ptashne and Gann 2002, 67). Many genes require for their differential activation the integration of a proper combination of several environmental signals, and this combination of signals, together with the presence of a particular combinations of activational factors, controls which exact sequence will be transcribed how much, and it will also effect cotranscriptional processes such as alternative splicing and RNA editing. Genes can therefore be expressed in many distinctive ways by different set of signals and activators. Not only because of these complicating factors of gene expression do we regard it as important not to downplay development as nothing but gene action and activation. Genes have an important role in development, but their role can be properly understood only within the larger system that holds controlling influence over them. Transgenerational stability need not rely on the faithful transmission of DNA. Genes depend on the context for their differential expression. Natural selection selects for adaptive traits or phenotypes, which are always derived from non-linear interactions among a range of diverse developmental resources. Their organization frequently exhibits phenotypic plasticity, a capacity that allows the organism to react adaptively to different environmental conditions (Pigliucci 2001; West-Eberhard 2003). The stable inheritance of this adaptive phenotype results from the reliable transmission of all the necessary developmental factors across generations. In other words, phenotypic plasticity relies on a stable ‘developmental niche’ which is faithfully reconstructed by the species, the parent and the organism itself. The subject of selection is the whole developmental system. 4. Extragenetic inheritance and developmental niche construction This construction of the developmental niche relies heavily on the extragenetic inheritance of developmental resources. This heterogeneous process includes maternal and paternal (parental) effects, which cannot be reduced to the influence of parental genes or RNAs on their offspring, but include all processes of care for the offspring. These are comprised of differential provisioning of resources, preference induction (oviposition, imprinting on food, habitat, and mates), and social learning, to name just a few (Jablonka and Lamb 2005; Mousseau and Fox 2003). Niche construction can be understood as one form of 'extended inheritance'. Inheritance systems have evolved to make the transmission of crucial information from parents to offspring more reliable. A reliably reproduced developmental system is the result of the reliable provision of a wide range of developmental resources necessary to reconstruct the organism’s life cycle, of which DNA is just one. Additional and equally necessary resources are DNA imprinting systems, cellular structures, ambient temperature, nutrients, gut organisms, and offspring care. Organisms have developed a range of strategies to construct the ontogenetic niche for their offspring to guide the developmental process. West and King were one of the firsts to “Ask not what’s inside the genes you inherited, but what your genes are inside of” (West and King 1987, 552). Looking at the enormous complexity of gene expression of eukaryotes that reveals a very flexible and reactive genome open to many intra-and extra-organismal environmental influences, it was simply a matter of time before some systems found ways to manage aspects of their own developmental environment. It is not so much the particular gene you inherit that counts but when, where and how a particular sequence is transcribed or translated by the higher order network of gene regulation that controls the time- and tissue dependent expression of genes. These mechanisms do not only control when genes are switched on and off, but also which parts of the DNA sequence will be transcribed, which will be spliced and in which combination, which will be edited at certain nucleotides, and which will be translated and at what rate. Some have referred to the particular mixture of gene products (protein transcription factors and RNAs) and the particular cellular signaling factors they react to as the cellular code. The cytoplasmic chemical gradients plus the messenger RNA and transcription factors that are inherited with the mother’s egg give this process a head start, but the mother’s control over the fetus’ environment does not stop there. Even after birth rearing practices, such as the licking of pups by rat mothers, continue to influence gene expression levels. The protein packaging of DNA can be modified in certain ways to influences which genes are transcribed. This imprinting system, often called the histone or chromatin code, gives the paternal and maternal genome control over the offspring’s gene expression. We can call the design-like control of the next generation’s developmental environment extended inheritance or just ontogenetic niche construction. Parental activity can facilitate, guide and entrench social learning, which in the case of humans and higher animals falls under the rubric of the cultural transmission of information. What all of these above cases of inheritance through environment construction have in common is making the transmission of crucial information more reliable. And while some of the above mechanisms have at first sight not much in common with the construction of epistemic structure by an extended mind, in the latter cases of behavioral, ecological and cultural inheritance the biological shades smoothly into the cognitive. There have been repeated attempts to reduce all of these mechanisms to the action of inherited or parent-of-origin genes, so that ultimately the real causes are all genetic. This special pleading fails in the light of the discovery that development relies less on the existence of genes in an organism than on the regulated expression of these genes, which ultimately depends on a host of environmental factors. Wherever there are genes there are extragenetic factors necessary for their regulated expression. 5. A new synthesis of epigenesis, evolution and extended heredity What a new account of development really has to accomplish is not just to go beyond these vexed dichotomies such as innate and learned, but to provide a framework that integrates a complex set of heterogeneous factors into a system of developmental resources all of which are reliably reproduced in succeeding generations of a developmental system but none of which really belong alone to either ‘gene’, ‘organism’ or ‘environment’ (the famous “Triple Helix” of Richard Lewontin 2000). Its contextualization of genes should obviate “even naïve temptations toward gene/environment dichotomies and … will open up a very rich area of empirical investigations to examination and conceptualization in developmental-system term” (Moss 2001, 85). The important systems features of such a view are the rejection of dichotomous description of behavior in favor of a full analysis in terms of continuing interaction between, and the joint determination by, heterogeneous developmental resources. Other important features are extending the idea of inheritance to include other factors than DNA, including factors formerly thought of as ‘environmental’ or ‘experiential’ if they are reliably reproduced or ‘passed on’ for succeeding generations; and last but not least the reconceptualization of evolution as (Oyama, Griffiths, and Gray 2001, 4). DST descibes evolution as construction in which evolutionary change results from the constructive interaction between all developmental resources, and between populations and their environments: the various elements of developmental systems coevolve. As we have seen organisms are not independent of or just passively dependent on their environments, they and their parental generation actively construct their developmental niches which is an integral part of the whole developmental system. References: Gilbert, Scott F. 2003. The reactive genome. In Origination of Organismal Form: Beyond the Gene in Developmental and Evolutionary Biology, edited by G. B. Müller and S. A. Newman. Cambridge, MA: The MIT Press. Jablonka, Eva, and Marion J. Lamb. 2005. Evolution in Four Dimenesions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. Cambridge, MA: The MIT Press. Keller, Evelyn Fox. 2000. The Century of the Gene. Cambridge, Mass.: MIT Press. Lewontin, Richard C. 2000. The Triple Helix: Gene, Organism, and Environment. Cambridge: Harvard University Press. Moss, Lenny. 2001. Deconstructing the gene and reconstructing molecular develomental systems. In Cycles of Contingency: Developmental Systems and Evolution, edited by S. Oyama, P. E. Griffiths and R. D. Gray. Cambridge, Mass.: MIT Press. Mousseau, Timothy A., and Charles W. Fox, eds. 2003. Maternal Effects as Adaptations. Oxford: Oxford University Press. Odling-Smee, F. John. 1988. Niche-constructing phenotypes. In The Role of Behavior in Evolution, edited by H. C. Plotkin. Cambridge, Mass.: MIT Press. Oyama, Susan, Paul E. Griffiths, and Russell D. Gray. 2001. Introduction: What is developmental systems theory? In Cycles of Contingency: Developmental Systems and Evolution, edited by S. Oyama, P. E. Griffiths and R. D. Gray. Cambridge, MA: MIT Press. Pigliucci, Massimo. 2001. Phenotypic Plasticity: Beyond Nature and Nurture, Syntheses in Ecology and Evolution. Baltimore: The Johns Hopkins University Press. Pigliucci, Massimo, and Jonathan Kaplan. 2007. Making Sense of Evolution: The Conceptual Foundations of Evolutionary Biology. Chicago and London: University of Chicago Press. Ptashne, Mark, and Alexander Gann. 2002. Genes and Signals. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Robert, Jason S. 2004. Embryology, Epigenesis and Evolution: Taking Development Seriously. Cambridge: Cambridge University Press. Stotz, Karola. forthcoming. 2001 and all that: a tale of a third science. Biology & Philosophy. West, Meredith J., and Andrew P. King. 1987. Settling Nature and Nurture into an Ontogenetic Niche. Developmental Psychobiology 20 (5):549-562. West-Eberhard, Mary Jane. 2003. Developmental Plasticity and Evolution. Oxford: Oxford University Press.