(Manufacturing Use Product). - Central Insecticides Board and
advertisement

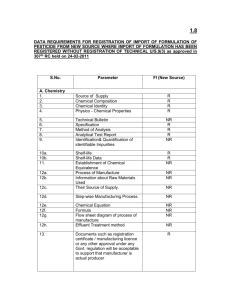
1.9 GUIDELINES / DATA REQUIREMENTS FOR REGISTRATION OF IMPORT OF MANUFACTURING USE PRODCUT (MUP) OF PESTICIDE U/S 9(3)-as approved in 316th RC held on 08-03-2011 Note:- 1. If the technical grade pesticide is not registered, complete data with respect to product technical on Chemistry, Bioefficacy, Toxicity and Packaging as per guidelines for technical Import (TI) U/S 9(3) to be submitted along with data on MUP as listed below. The technical shall be deemed registered after expiry of 3 years from the date of registration of MUP & its formulation u/s 9(3). 2. If the technical grade and source for import is duly registered as per guidelines of the Registration Committee, data on MUP to be submitted as listed below. 3. Rationale for import / registering the MUP-to be submitted in Chemistry, efficacy, Toxicity and Packaging folders. S. No A. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Bio- Parameter Chemistry Source of Supply Chemical Composition Chemical Identity Physico - Chemical Properties Technical Bulletin Copy of RTT permit Specification Method of Analysis Analytical Test Report Shelf-life claim Shelf-life Data Documents such as registration certificate / manufacturing licence or any other approval under any Govt. regulation will be acceptable to support that manufacturer is actual producer Certificate from manufacturer that the dealer/ trader is an authorized dealer/ trader of the manufacturer. A test report about the quality of the product from a laboratory as per GLP scheme or from a company of ISO-9000. This requirement will be provided along with first consignment. Thereafter, each consignment should have proper analytical test report of the manufacturer. The applicant should provide sample along- with standards technical sample from the principals/ authorized dealers for chemical verification. In case of technical grade pesticides u/s 9(3), samples of std. impurities are also to be provided for chemical verification. B. 1. 2. 3. 4. C. 1. 2. 3. 4. 5. 6. 7. 8. Bioefficacy Persistence in soil Persistence in water Persistence in Plant Registration status of Manufacturing use product (MUP) in foreign countries Toxicity Acute oral rat & mice Acute dermal Acute inhalation Primary skin irritation Irritation to mucous membrane Acute Toxicity to Birds (Two Species) Acute Toxicity to Fish Acute Toxicity to Honeybees D. Packaging 1. 7 copies of Labels / Leaflets (Ls/Ls) for small size container up to 250ml capacity and 7 copies of Ls/Ls for large size container above 250ml capacity. Type of packaging Information about packaging material proposed of the product in retail or bulk. Compatibility data as per relevant IS standard. Information / data on suitability of the proposed packaging system for protecting the content through- out the shelf life. Past registration data can also be submitted as per shelf life studies of the product. Information on the proposed packaging material regarding its incorporation in IS standard. 2. i ii iii iv 3. i ii 4. 5. i ii iii 6. 7. Manner of packaging Information regarding filling, sealing of closure system, pilfer proofness, leakproofness on primary, secondary and transport packaging. Undertaking for the use of pilferproof, leakproof and easily disposable container of the packaging of the product. Transportation: undertaking for safe storage handling and transportation of the product. Manner of labelling Information as per provisions of section 18 & 19 of Insecticides Rules, 1971 Instructions for storage and use including precautionary measures. Instruction regarding disposal of used material and washing of Insecticides should be proposed. Transport Worthiness Test report for the proposed packaging system. Performance of containers report during the storage stability tests.