genomic equivalence and the cytoplasmic environment [07]
advertisement
![genomic equivalence and the cytoplasmic environment [07]](http://s3.studylib.net/store/data/007302398_1-6520ba79235faff972bb689b154d5575-768x994.png)
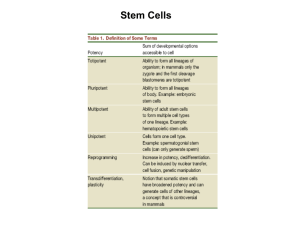
BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF PRIMER ON STEM CELLS AND RELATED TOPICS Regenerative medicine encompasses several new areas of medical research seeking to treat diseases or injuries by transplantation of cells/tissues/organs grown in vitro or by stimulating the patients’ own capabilities for regeneration. The underlying biological topics include stem cells, cell determination, cell differentiation, epigenetic changes, and related phenomena, which will be reviewed here. Regeneration will be taken up separately. A. Adult Stem Cells 1. Development entails formation of differentiated cells from embryonic or other undifferentiated cells. This process can be monitored by a. cell morphology and location b. specific molecular markers, such as transcription products or proteins 2. A large mammal may have trillions of cells, but only about 200 different cell types. 3. Most differentiated cells have limited life spans and do not divide. Instead, worn out cells are replaced by from reserve cells called “stem cells” [ABD 0311]. 4. The definition of adult stem cells uses the following criteria [1PPE, HB 10.6] a. undifferentiated appearance (cuboidal cell shape, large nuclei) b. unlimited capacity for mitotoic division c. Two types of daughter cells: new stem cells and committed progenitor cells 5. Examples of adult stem cell lineages include: epidermal cells, intestinal epithelial cells, blood cells, mammalian spermatogonia, Drosophila oogonia. 6. Adult stem cells may be a. unipotent, i.e. giving rise to only one type of differentiated cells, such as epidermal cells or spermatogonia. b. multipotent, i.e. they normally give rise to two or more types of differentiated cells. Examples: hemangioblast, universal blood stem cell [ABD 20.12] 7. The stem cell niche is defined as the microenvironment of extracellular signals that maintain “stemness” in a stem cell lineage. Such signals may be a. secreted and diffusible (e.g. TGFß or Wnt families) b. integral membrane proteins (e.g. Notch and Delta) c. integrins and extracellular matrix components. Their stiffness and adhesiveness give them an astounding degree of control over the release of committed progenitor cells from their niche. 8. The asymmetry of stem cell divisions may occur in two basic forms [WH Fig. 1] a. “invariant” asymmetry depending on organelles orienting mitotic spindle in conjunction with localized cytoplasmic determinants or unevenly distributed external signals). Example: germ line stem cells in Drosophila ovary b. “populational asymmetry” producing stem cells and committed progenitor cells stochastically (depending on labile control circuits of transcription factors and extracellular signals) 9. Under experimental conditions, some adult stem cells have been shown to form differentiated cells beyond their normal, tissue-specific repertoire a. mouse neural stem cells formed blood cells b. bone marrow-derived stromal cells, aka mesenchymal stem cells (MSC), gave rise to cardiomyocytes and epithelial cells in lung, liver, and skin. 137\STEMCELLS_ETC\12Sp 1 BIOLOGY 137 B. HUMAN BIOLOGY SEMINAR K. KALTHOFF Embryonic Stem Cells 1. Mammalian embryonic stem cells (ESC) are defined by the following operational criteria. a. they are obtained from the inner cell mass (ICM) of mammalian embryos at the blastocyst stage [ABD 5.8, 5.11, 5.12] b. they fulfill the defining criteria used for adult stem cells (section A4) c. they are highly pluripotent, that is, capable of forming a wide (perhaps unlimited) range of differentiated cell types. This was shown by the i. formation of teratomas in vivo, after transplantation into normal animals ii. ability to form, in vitro, differentiated cells normally derived from any germ layer (ectoderm, mesoderm, endoderm) depending on culture conditions d. Mouse ESC are totipotent, i.e., capable of forming complete, fertile embryos as shown by the tetraploid complementation test [HB 10.7]. It is generally assumed that human and other mammalian ESCs are also totippotent. 2. ESC not only satisfy the defining criteria of adult stem cells but exceed them in a. ease of identification b. robustness and rapid division in culture c. pluripotency if not totipotency 3. For basic research, the culture of ESC is an excellent way of studying the signals that gradually turn embryonic cells into differentiated cells. 4. For applied research, ES cells are prized because of their a. use in making transgenic animals by homologous recombination in cultured ESC followed by transplantation into host blastocysts b. potential to generate isogenic human replacement cells in conjunction with nuclear transfer and/or gene therapy (see Section D) 5. Human ES cells are derived from blastocysts generated in fertility clinics, which routinely fertilize more eggs in vitro than can be used for one uterine implantation. The spare blastocysts are deep-frozen and can be used for additional implantations as needed. Otherwise these blastocysts are spare and could be used for research. 6. Research on human embryonic stem cells is politically controversial, and federal funding in the U.S. has changed under different administrations. The British Parliament has passed laws including a “14-day rule”. Up to this time limit, approved research on human embryos is legal. In fact, there are incentives for British IVF parents to make their spare embryos available for research. C. Totipotency of Animal Blastomeres 1. A fertilized egg (zygote) undergoes cleavage, i.e. a series of cell divisions generating progressively smaller cells called blastomeres. 2. In many animals including mammals, blastomeres and are totipotent, as shown by blastomere isolation and monozygotic twin formation [ABD 0114, 0601] 3. In many animal species, blastomeres can also depart from their normal fates in development, an amazing ability called regulation. It is shown by the same blastomere isolation experiments and by fusion of multiple embryos [ABD 0623]. 137\STEMCELLS_ETC\12Sp 2 BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF 4. The ability of human embryos to undergo regulation is utilized in the procedure called preimplantation genetic diagnosis, which is offered by fertility clinics as an adjunct to in vitro fertilization. At the 8-cell stage of development, one blastomere is removed for genetic testing before the remainder of the embryo is implanted. Apparently, the procedure does not affect the chances of a successful pregnancy. 5. Attempts to regenerate whole organisms from single (or small groups of) differentiated cells have been successful in plants, sponges and hydrozoans but not in higher animals. This experience has been ascribed to restrictive effects of animal cytoplasm and prompted studies using nuclear transfer into eggs. Such restrictive effects were thought to be minimal in eggs because eggs normally support the development on an entire embryo. D. Nuclear Transfer into Eggs 1. Nuclear transfer into surgically enucleated eggs of Rana pipiens [ABD 0712] a. Nuclei from differentiated blood cells, intestinal cells, spermatogonia, and other adult cells have supported the development of complete organisms, including a few adults [ABD 0715], but the success rate was uniformly low. b. Nuclei from older donor cells show a decreasing ability to promote the development of an entire organism [ABD 0713]. c. Conclusion: Nuclei from at least some differentiated cells are pluripotent or even totipotent. However, chromatin from differentiated cells may need some "remodeling" for DNA replication and new embryonic gene activities. 2. In sheep, a cultured fibroblast was fused with an enucleated egg, which then was implanted into the uterus of a foster ewe and born as an apparently normal lamb (“Dolly”) [ABD0717]. Similar experiments with cows, pigs, goats, cats, dogs, mice and Rhesus monkeys, using nuclei from various types of donor cells. 3. Failure rates have been consistently high in sheep [ABD Table 7.1] as well as in other mammals, and have been shown to increase rapidly during early development in mice [1 PPE]. These high failure rates preclude the use of nuclear transfer for human reproductive cloning. 4. However, nuclear transfer should be pursued for therapeutic cloning, i.e. the generation of human ESC lines isogenic with patients who may be cured by replacing a particular lineage of deficient cells. 5. In a futuristic scenario, patients with type I diabetes, Parkinson’s disease, and similar disorders affecting one particular cell type could be treated by cell replacement therapy [HB 10.11]. A small biopsy from the patient would be cultured to obtain cells that can be used as nuclear donors when fused with an enucleated human egg. When they reach blastocyst stage they are sacrificed to obtain the inner cell mass (ICM). About 10% of ICM cultures yield embryonic stem cells (ES cells), which are undifferentiated, multiply indefinitely, and are pluripotent [HB 10.6]. ES cells are exposed to appropriate growth factors and ECM stimuli to obtain the desired type of committed progenitor cells. These are transplanted to the patient. 6. The major advantage of this method is that the replacement cells are isogenic (genetically identical) with the patient, so that no immune rejection will occur. Another advantage is that the method could readily be combined with gene therapy in 137\STEMCELLS_ETC\12Sp 3 BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF cases where the human disease is caused by a defect in a known gene. A proof-ofprinciple experiment with mice has already been done [HB11.6]. 7. A major concern is that the transplanted progenitor cells may be contaminated with residual ES cells, which could form tumors. Another problem is that the method requires large numbers of donated human eggs because only a fraction of re-nucleated eggs develop into blastocysts and only a fraction of those give rise to ESC cultures. Such low yields drive up the cost of cell replacement therapy and exacerbate ethical concerns about using human eggs and blastocysts for research. 8. This dilemma highlights the need to better understand why it has been so difficult to return differentiated mammalian cell or their nuclei to a totipotent or at least pluripotent state. What is the nature of the “chromatin remodeling” that seems to be required? E. Two Ways of Chromatin Remodeling 1. The basic tenet that all cells of an organism are isogenic is holding up as a general principle. DNA losses, selective replication, or rearrangements occur in some cell lineages, but these seem to be exceptions rather than the rule. Cell differentiation must therefore be ascribed to differential gene expression [ABD 7.2]. 2. In accord with the principle of differential gene expression, cell determination and cell differentiation always involve the deployment of specific transcription factors. 3. Transcription factors act by binding to specific promoter and enhancer sequences. However, such binding may be restricted by the way nuclear DNA is packaged with histones (nucleosomes) and other chromosomal proteins [HB 10.4]. 4. The genes that are most actively transcribed in cells, such as globin genes in red blood cells or ovalbumin genes in oviduct, coincide with those chromosomal regions that are most sensitive to mild digestion with DNase I. 5. Conceivably, chromatin could be “remodeled” by changing a. the density of chromatin packaging, which would render the DNA more or less accessible to transcription factors. Related observations are covered in Sections to F to H. b. the availability of transcription factors, which could displace histone proteins. This seems to be the case in induced pluripotent stem cells (see Section I). c. both remodeling processes are based on the dynamic nature of protein binding to DNA. F. Genomic Imprinting Several observations highlight the importance of semi-permanent modifications of DNA and histones that change the packaging of chromatin in eukaryotic cells. 1. One line of related observations was made in pronuclear transplantation experiments with newly fertilized mouse eggs [ABD 4.16]. a. Natural parthenogenesis is unknown in mammals. b. Unfertilized and artificially activated mouse eggs die halfway through gestation. c. Experimental transfer of pronuclei [ABD Table 4.2] can generate zygotes with 137\STEMCELLS_ETC\12Sp 4 BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF i. two female pronuclei but no male pronucleus. They develop into bimaternal embryos, which cease development about the same time as parthenogenetic embryos ii. two male pronuclei but no female pronucleus. They develop into bipaternal embryos, which also die midway through gestation but show defects different from bimaternal embryos iii. one male and one female pronucleus, which develop normally d. These results indicate that male and female pronuclei contribute some gene activities to the fertilized egg (zygote) that cannot be substituted by the homologous genes in the pronucleus from the other sex. 2. Another set of observation was made by human geneticists on patients with uniparental disomy (UPD), who have inherited two copies of a chromosome from one parent and no homolog from the other parent. a. patients with maternal UPD 15 (two chromosomes 15 from mother, no chromosome 15 from father) show Prader-Willi syndrome, which includes severe obesity, gonadal dysfunction, short stature, and mental retardation. b. Paternal UPD 15 causes Angelman syndrome, which includes seizures, sleep disorder, severe mental retardation, and lack of speech. 3. These and other observations indicate that there is a sex-specific and reversible silencing of certain autosomal genes in mammals known as genomic imprinting or DNA imprinting [ABD 16.24]. a. Some genes (about 30-40) are imprinted during spermatogenesis while others are imprinted during oogenesis. b. The imprinted genes remain silent in early embryonic and in all somatic cells, including the brain. The homologous genes are then expressed uniparentally. c. The imprinting is erased in each generation’s primordial germ cells, so that germ line cells express both alleles of previously imprinted genes. d. During gametogenesis, each parent imprints anew the sex-specific set of genes. 4. The ultimate cause of genomic imprinting seems to be a conflict of the sexes over the amount of nutrients that mammalian mothers transfer to their offspring. Females imprint genes that would otherwise favor a developing fetus at an undue cost to the mother. Conversely, males imprint genes that would otherwise limit the amount of maternal nutrients received by the offspring. G. DNA Methylation, Histone Acetylation 1. The mechanisms of imprinting are incompletely understood but seem to involve DNA methylation and histone acetylation. 2. In more than half of the CpG dinucleotides in mammalian DNA, the C residue is modified with a methyl group to form 5-methyl cytosine. An existing methylation pattern in CpG dinucleotides is propagated during DNA replication by an enzyme called DNA (cytosine-5) methyltransferase I (Dnmt 1) [ABD 16.23]. 3. DNA methylation in promoter regions generally inhibits gene expression. 4. At least one way in which DNA methylation exerts its inhibitory effect is by histone deacetylation. A protein known as methyl CpG binding protein 2 (MeCP2) binds to methylated CpG and associates with the enzyme histone deacetylase (HDAC). 137\STEMCELLS_ETC\12Sp 5 BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF 5. HDAC in turn removes an acetyl group from lysine residues of histone, rendering the histone more basic and thus more tightly binding to DNA [ABD 16.18]. Tighter binding to histone makes DNA less accessible to transcription factors. 6. The opposite reaction is catalyzed by histone acetyltransferase (HAT). H. Concept and Key Methods of Epigenetics 1. DNA methylation and histone modifications do not change the DNA nucleotide sequence, but rather the accessibility of chromosomal DNA for gene-regulatory proteins [Tsankova et al. (2007a, Fig. 1a,b]. Nevertheless, the changed chromosomal states and the resulting modifications in gene expression are passed on during cell division. Hence these mechanisms have been called epigenetic (Greek epi: over, above). Remarkably, epigenetic states can be affected by behavioral cues and by drugs, and as these are passed on between generations, so are the resulting epigenetic states. In this sense, epigenetics can amount to a “soft inheritance” of acquired traits. 2. For example, happy rat mothers lick and groom their pups, and their female offspring in turn grow up to be high licking/grooming mothers [HB 13.6]. Characteristically, the mothering style is passed on to both biological and adoptive daughters. Molecular analysis has shown that high-care mothering activates the gene for glucocorticoid receptor (GR) in the pups’ hippocampus. The activation was found to be mediated by DNA demethylation and histone acetylation in the promotor region of the hippocampal GR gene. Most importantly, a drug inhibiting histone deacetylase (thus promoting histone acetylation) changed the behavior of rats raised by low-care mothers towards the behavior normally shown by offspring of high-care mothers. 3. The role of epigenetic changes in development was conceptualized by Conrad Hal Waddington (1905 – 1975) as an epigenetic landscape [Waddington landscape]. It shows marbles rolling down a hill with a bifurcating series of valleys, each fork representing a change in gene expression and in the resulting differentiated cell type. The hills between the valleys represent the difficulty encountered in experiments involving regeneration or nuclear transplantation, which try to change the series of bifurcation points that the cell has already passed. 4. The key method for detecting methylated cytosine residues in genomic DNA is known as methyl mapping. It uses sodium bisulfite treatment to convert unmethylated cytosine to uracil, which is detected by sequencing. (Methylated cytosine resists the conversion to uracil.) 5. The key method for detecting histone modifications and DNA binding proteins is known as chromatin immunoprecipitation (ChIP). It aims to determine whether certain genomic regions are associated with specific proteins, such as transcription factors or modified histones. Briefly, protein and DNA are cross-linked before the chromatin is sheared. The DNA fragments cross-linked to the protein(s) of interest are immunoprecipitated, and then the associated DNA fragments are sequenced. 6. These methods are rapidly being developed for high throughput with the goal of mapping epigenetic changes over the entire genome in various healthy and diseased tissues at different stages of development, in primary tumors, etc. 137\STEMCELLS_ETC\12Sp 6 BIOLOGY 137 HUMAN BIOLOGY SEMINAR K. KALTHOFF I. Induced Pluripotent Stem Cells A different approach to studying chromatin remodeling and cell differentiation has been taken by providing cells with extra amounts of key transcription factors. 1. Early during the analysis of skeletal muscle differentiation, it was found that cultured connective tissue cells could be converted to myoblasts (committed skeletal muscle precursor cells) by adding to the culture medium certain transcription factors, such as MyoD. From such experiments and the phenotypes of knock-out mice, an entire hierarchy of transcription factors driving the differentiation of skeletal muscle fibers was constructed. 2. A similar approach was used in a pioneering experiment on transcription factors that confer “stemness” to cultured fibroblasts and other differentiated cells. In a pioneering line of work, Shinya Yamanaka and coworkers at Kyoto University [Photo Yamanaka] transformed differentiated cells into induced pluripotent stem cells (iPS cells), which resemble embryonic stem cells (ES cells). a. Using retroviral vectors, they added to the genomes of target cells extra copies of genes that are regularly expressed in ES cells [Hochedlinger and Plath, 2009]. b. In the original experiment, they tested 24 candidate genes on skin cells (fibroblasts) from mice. A combination of four genes encoding different transcription factors, namely Oct 3/4, Sox 2, c-Myc, and Klf4, was successful in conferring “stemness”, as judged by embryonic morphology, unlimited division, and pluripotency. 3. Follow-up experiments were aimed at avoiding the oncogenic effects of some of the stemness-inducing transcription factors and the disruptive effects of transgene insertion by viral vectors. a. Yu et al. (2007) obtained iPS cells by transforming human fibroblasts with a combination of genes (Oct4, Sox2, Nanog, and Lin28) that did not include c-Myc. This result was significant because c-Myc is notorious as a proto-oncogene. b. Woltjen et al. (2009) and Kaji et al. (2009) built on the observation that the stemness-inducing transcription factors need to act only temporarily. Using a known inducible recombination enzyme, they seamlessly removed the transgenes after they had done their stemness-inducing work. c. Manos et al. (2010) used synthetic RNAs corresponding to the four classical iPS cell transgenes to transform multiple human cell types into ES-like cells. The authors call these cells RNA-induced pluripotent stem cells, or RiPS cells. The transforming RNA is quickly degraded. 4. Mouse and human iPS cells were shown to be pluripotent in vivo by their ability to form teratomas when injected into the skin of mice, and in vitro by culture in the presence of different growth factors. Mouse iPS cells were also shown to be totipotent by the tetraploid complementation assay (section B1d). 5. In human medicine, iPS cells hold much promise for several reasons. a. Patient-specific iPS cell cultures can be used as models for studying human diseases and as preliminary screens for therapeutic drugs [Science 29 Aug. 2008]. b. iPS cells are considered to be great candidates for cell replacement therapy because they can be generated from the patient’s own cells, so that there will be no immune rejection. 137\STEMCELLS_ETC\12Sp 7 BIOLOGY 137 6. 7. 8. 9. HUMAN BIOLOGY SEMINAR K. KALTHOFF c. If the need for therapy is due to a genetic disorder, the defective gene can be replaced by homologous recombination in iPS cell culture. d. As compared to making ES cells isogenic by nuclear transfer, iPS cells do not depend on the availability of donated human eggs. Working with iPS cells also avoids ethical concerns about sacrificing human blastocysts. e. However, because of their more recent discovery, iPS cells are currently further away from clinical applications than ES cells. And some recent observations have raised concerns. i. Lister et al. (2011) observed that the methylation patterns of iPS cells retained some “memories” of the adult cells from which they had been derived. ii. Laurent et al. (2011) found that human ES cells as well as iPS cells acquired gene duplications and deletions during culture. Thus, returning differentiated cells, or their nuclei, to a pluripotent state for cell replacement therapy requires genomic and epigenomic monitoring to ensure stability and safety. The relative ease of making iPS cells could hasten attempts to derive gametes from such cells. Corresponding experiments to make mouse sperm from iPS cells are well under way (Okita et al., 2007). Established in vitro fertilization procedures could then be used to produce human babies, and certain infertile couples will probably be eager to avail themselves of this way to have children. There is concern that genetically modified human iPS cells may be used to generate gametes, and thus, genetically “enhanced” human babies. This prospect raises great concern because many human genes are pleiotropic, and the newly created genotypes would not have been tested in evolution. At the behest of Shinya Yamanaka, the principal investigator in the original iPS cell study, the Japanese science ministry notified all universities and research agencies not to produce gametes from iPS cells and not to implant embryos made with iPS cells into human or animal wombs. Patent applications that would make the unauthorized use of iPS cell technology illegal are also under way. 137\STEMCELLS_ETC\12Sp 8