Moving Moles - Double Dilution
advertisement

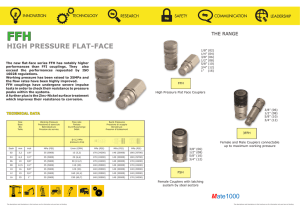
Example Calculation Example Problem: You are working in the lab and your PI asked you to set-up a PCR reaction. You have 500 l of a 50 nM stock solution of primers and need 10l of a 10 pM working stock. A- Do you have a sufficient amount of stock reagent to do it? B- If so how would you do it? Solution to Problem: 1) Write down the numerical relationship between moles, mmoles , moles, nmoles, pmoles and fmoles. They all differ from each other by 1000 right? 2) Determine what you have and what you need from the problemYou have 500 l of a 50 nM stock solution You need 10l of a 10 pM working stock 3) Figure out your dilution factorYou must make the following dilution 50nM 10pM which is a 1:5000 How do you know that? Well, 50nM = 50,000pM and 50,000/10 = 5000 So your dilution factor (DF) is 5000 and thus the dilution of your stock solution that is needed is 1:5000 Said another way, 50,000 pM is 5000 times greater than 10 pM. So you must dilute the 50,000pM solution 5000 times to make a 10 pM solution. 4) Determine if you can make this dilution directly in the volume you needRemember what you have and what you need from the problem (i.e. You have 500 l of a 50 nM stock solution and you need 10l of a 10 pM working stock) So dilute the volume you need (i.e. 10 l) by your DF (i.e. 5000) 10/5000= 0.002l Can you measure that with a micropipetor? If you can just do that in one step ! If no…you have to do a tweenzie (intermediate) dilution. The trick to the tweenzie is that your final DF is 5000 so you dilute your stock twice and both dilutions multiplied together must equal 5000. How you do that is your decision. In this example, it could be 1:2 and 1:2500 (2 x 2500 = 5000) or 1:10 and 1:500 (10 x 500 = 5000), etc. Here we will do 1:100 and 1:50 (100 x 50 =5000; see figure below). 1:100 1:50 1 l stock + 99 l solvent Stock (50 nM) 1 l tweenzie + 49 l solvent Tweenzie (0.5 nM) Final Working Stock (10pM) 1:100 means 1 part stock in a total of 100 parts. How you actually do the dilution is up to you. So a 1:100 is 1 l stock + 99 l solvent (say water in our case). It could also mean 2 l stock + 198 l solvent. How do you know that? 200/2 = 100 As shown below the arrows above we did 1 l + 99 l of solvent. A 1:50 means 1 part in a total of 50 parts. So a 1:50 is 1 l stock + 49 l solvent. It could also mean 2 l stock + 98 l solvent. How do you know that? 100/2 = 50 As shown below the arrows above we did 1 l + 49 l of solvent So you have sufficient reagent to do it, and thus it can be done! And that is not always the case!!! But how do you know you did it right? Remember you are moving moles around. Compare what you need to what you deliver. A- What you need10 pM = 10 pmoles/L so walk it down 10 pmoles/L = 10 fmoles/ml = 0.010fmoles/l What you do to the numerator you do to the denominator to keep everything equal. Notice all of the numerators and denominators are 1000 times different, for example you go from pmoles to fmoles in the numerator. You also go from L to ml in the denominator! OK so--- 0.010fmole/l X 10 l = 0.1 fmoles needed B- What you delivered50 nM = 50 nmoles/L so walk it down too 50 pmoles/ml = 50 fmoles/l And remember you diluted your stock solution 1:5000 So 50 fmoles/l = 0.010 fmoles/l 5000 OK so-- 0.010 fmoles/l X 10 l = 0.1 fmoles delivered If 0.1 fmoles was needed and 0.1 fmoles was delivered you did your calculation right!!