Irrigation Materials
advertisement

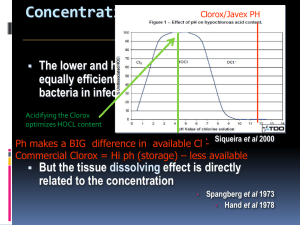
Irrigation Materials Instrumentation of the root canal system must always be supported by an irrigation system capable of removing pulp tissue remnants and dentin debris. In modern treatment systems the irrigation fluid is delivered with a fine-caliber needle in large volume, and the debris is aspirated with a good suction device. The effervescence created by mixing NaOCl with hydrogen peroxide has been used to remove debris from the root canal, but this is not an effective method. Liberal irrigation is essential for effective functioning of the files. Many anecdotal descriptions of the lubricating effect of irrigation fluids are merely the mistaken effects of debris transportation. Without irrigation, instruments rapidly become ineffective because of the accumulation of debris. Irrigation also is essential for reducing the number of bacteria in an infected root canal, but it has only a minimal antimicrobial effect on the infected root canal walls. Consequently, the antimicrobial effect of an irrigation fluid should not be the clinician’s only concern when choosing among suitable compounds. Surface tension and cleaning effectiveness are equally important qualities. Quaternary ammonium compounds with a low surface tension have been extensively used as irrigation fluids. These fluids are detergents and therefore effective aids in pulp space cleaning because they remove lipid pulp breakdown products. However, they now are rarely used because of their toxicity. Quaternary ammonium compounds are still used as additives to ethylene diamine tetra-acetic acid (EDTA) in EDTAC (EDTA and cetrimide) to provide some antimicrobial effect. NaOCl has become the irrigant of choice worldwide. Its strong proteolytic effect makes it an excellent aid during instrumentation. Chlorhexidine has also been suggested as an irrigant, but it has few advantages over NaOCl during instrumentation. A 2% solution of chlorhexidine has proved more effective against Enterococcus faecalis than sodium hypochlorite. Chlorhexidine also differs from sodium hypochlorite in that it is relatively nontoxic and does not dissolve tissue. If applied to dentin, it binds effectively to hydroxyapatite, providing a lasting reservoir of chlorhexidine after the completion of treatment. Some have suggested that this long-term effect may be helpful in reducing the effect of postoperative coronal leakage. One study showed that combined use of chlorhexidine and sodium hypochlorite resulted in a greater percentage of microbe reduction than was achieved with either used alone. New irrigants are being developed in an attempt to address some of the shortcomings of past and current materials. MTAD is a mixture of a tetracycline isomer (i.e., doxycycline), an acid, and a detergent. In an in vitro study, MTAD was found to be an effective solution for killing E. faecalis. Proteolytic Materials The most commonly used proteolytic irrigation material is NaOCl, which became an important agent for the treatment of infected wounds in the early twentieth century. NaOCl dissolves necrotic tissues and debris through a complex biochemical process. The amount of free chlorine is important for this breakdown of proteins into amino groups. Higher temperatures also potentiate the antimicrobial and tissue-dissolving effects of NaOCl. The original concentration suggested by Dakin was 0.5%, but concentrations as high as 5.25% have been used in dentistry. A 1% concentration provides sufficient tissue dissolution and antimicrobial effect if used freely. Higher concentrations of NaOCl affect living tissue and do not improve the reduction of bacteria during endodontic treatment. NaOCl has an antimicrobial effect as long as free chlorine is available in the solution. Because free chlorine is the important component consumed during tissue breakdown, the NaOCl must be replenished frequently, especially when low concentrations are used. This becomes even more important when the root canals are narrow and small. Sodium hypochlorite does not effectively wet dentin, and small canals and canal extensions are poorly irrigated. Attempts have been made to change the surface tension of NaOCl but without significant success. NaOCl also has been shown to deplete dentin of organic compounds and to increase the permeability of dentin significantly. Pure NaOCl is a U.S. Pharmacopeia (USP) preparation and may be purchased from a pharmacy. However, dentists commonly use commercial 5.25% NaOCl (i.e., household bleach). At this concentration NaOCl is highly toxic, meaning that it unnecessarily necrotizes wound surface areas that should remain unharmed. The literature widely suggests that postoperative pain is no greater when high concentrations of NaOCl are used. However, this lack of correlation proves little, because tissue damage and clinical symptoms are poorly correlated. Commercial NaOCl is buffered to a pH of approximately 12 to 13. This adds another toxic component, making the solution even more caustic. Therefore if commercial bleach is used as a base for preparing a 1% irrigation solution, it is better to use sterile 1% sodium bicarbonate as a diluent rather than water. This helps to adjust the pH to a less caustic level. Diluted, buffered NaOCl has a limited shelf life and should be stored in a dark, cool place for no longer than 1 to 2 weeks. Few clinical complications are associated with the use of NaOCl. The most common one is accidental injection of NaOCl into periradicular tissue. This results in excruciating pain, periapical tissue bleeding, and extensive swelling. The pain normally subsides within 2 to 3 days. The swelling increases for the first day, after which healing occurs. The prognosis is usually good if no critical tissues, such as the mental nerve, have been damaged. Sodium hypochlorite and hydrogen peroxide are known to release oxygen-free radicals and have the potential to reduce the bonding of resin to dentin. Alcohol, chlorhexidine, saline, EDTA, anesthetics, and sodium ascorbate do not seem to affect the bond strength of resin, and they may even remove or negate the oxygenfree radicals. One study found that short-term use of calcium hydroxide Ca(OH)2 did not affect dentin bond strengths when ethanol or acetone-based adhesive resin was applied. Detergents Detergents are often used as irrigation solutions because they are effective at removing the fatty tissue residues that are byproducts of tissue necrosis. Commonly used materials are included in the family of quaternary ammonium compounds. These compounds were once considered optimal for antimicrobial therapy and effective in very low concentrations. However, this has been disproved, and the preparations have been shown to have a toxicity comparable with that of other irrigation solutions and a rather narrow bactericidal spectrum.Quaternary ammonium antiseptics normally are used in a water solution at 0.1% to 1%. Zephiran chloride has been commonly used as an endodontic irrigation solution. However, in light of its toxicity and low antimicrobial effectiveness, no reasons exist to use this detergent instead of a relatively less toxic irrigating solution (i.e., 1% or less NaOCl solution). Another group of antimicrobial agents with detergent effects are the iodophores. Wescodyne and Iodopax are common products in this line of antiseptics. These organic iodine products are effective at low concentrations. They are antimicrobially effective at an iodine concentration of 0.05% (volume/volume). Detergents have also been mixed with calcium hydroxide for irrigation. Decalcifying Materials A smear layer is formed during preparation of the root canal. No clear scientifically based understanding exists on whether this layer must be removed or can be left. However, a multitude of opinions have been offered on both sides of this question. In addition to weak acids, solutions for the removal of the smear layer include carbamide peroxide, aminoquinaldinium di-acetate (i.e., Salvizol), and EDTA. In objective studies, carbamide peroxide and Salvizol appear to have little effect on smear layer buildup.A 25% citric acid solution also failed to provide reliable smear layer removal. EDTA is often suggested as an irrigation solution because it can chelate and remove the mineralized portion of smear layers. It also can decalcify up to a 50 μm layer of the root canal wall if used liberally. EDTA is normally used in a concentration of 17%. It removes smear layers in less than 1 minute if the fluid is able to reach the surface of the root canal wall. Reports suggest that under clinical conditions, the fluid should be kept in the root canal for at least 15 minutes for optimal results. The decalcifying process is self-limiting, because the chelator is used up. To achieve continuous effect, the EDTA must be replaced through frequent irrigation. For root canal preparation, EDTA has limited value as an irrigation fluid. It may open up a hair-fine canal if given the time to soften the 50 μm it is capable of decalcifying. This amount, at two opposite canal walls, results in 100 μm. This is equivalent to the tip of a #010 file. The smear layer consists of both an organic and an inorganic component. EDTA alone normally cannot remove the smear layer effectively; a proteolytic component (e.g., NaOCl) must be added to remove the organic components of the smear layer. Commercial products with such combinations are available. EndoDilator N-Ø (Union Broach, York, PA) is a combination of EDTA and a quaternary ammonium compound. Such an irrigation fluid has a slight detergent effect in addition to the chelating effect. Two newer irrigating solutions, MTAD (Dentsply–Tulsa) and Smear Clear (SybronEndo), have recently been studied. Smear Clear, which is commercially available, is a clear, odorless, water-soluble solution containing water, 17% EDTA salts, a cationic surfactant (centrimide), and anionic surfactants. Intracanal Disinfection Materials Biomechanic instrumentation and irrigation with an antimicrobial solution are essential for disinfection of the pulp space, but some suggest that these techniques may not completely eradicate microorganisms in a necrotic pulp space and that further disinfection with an effective antimicrobial agent may be necessary. Phenol and phenol derivatives are the most commonly used intracanal disinfectants. Antiseptics with a chlorine or iodine base are also common. In recent years more attention has been given to the use of calcium hydroxide as an intracanal dressing for the treatment of infected pulp necrosis. Conventional antiseptics generally are toxic, and care must be taken not to cause undue tissue damage. Phenolic Preparations Phenol (C6H5OH), or carbolic acid, is one of the oldest antimicrobial agents used in medicine. Despite the severe toxicity of phenolic preparations, derivatives of phenol, such as paramonochlorophenol (C6H4OHCl), thymol (C6H3OHCH3C3H7), and cresol (C6H4OHCH3), remain available. One survey noted a decrease in the use of classic phenolic medicaments with a corresponding increase in the use of calcium hydroxide or no medication. Phenol is a nonspecific protoplasm poison that has an optimal antibacterial effect at 1% to 2%. Many dental preparations use much too high a concentration of phenol (e.g., in the range of 30%). At such a concentration the antimicrobial effect in vivo is lower than optimal and of very short duration. Derivatives of phenol are stronger antiseptics and toxins than phenol. Phenolic compounds are often available as camphorated solutions. Camphoration results in a less toxic phenolic compound because it slows the release of toxins to the surrounding tissues. Studies in vitro have shown that phenol and phenol derivatives are highly toxic to mammalian cells and that their antimicrobial effectiveness does not sufficiently balance their toxicity. Experimentation in vivo also demonstrated that phenol and phenolic derivatives induce inflammatory changes at much lower concentrations than many other antimicrobial agents. Phenols are ineffective antiseptics under clinical conditions. In one study, 2 weeks of intracanal dressing (in which the canals were filled with camphorated phenol or camphorated parachlorophenol) failed to eliminate intracanal bacteria in one third of the cases. Phenolic compounds are also unable to release an effective antimicrobial vapor and therefore are ineffective when placed on a cotton pellet in the pulp space. Formaldehyde Formaldehyde has been used extensively in endodontic therapy despite its high toxicity and mutagenic and carcinogenic potential. The compound of interest when discussing pulp space disinfection is formocresol. The formaldehyde component of formocresol may vary substantially between 19% and 37%. Tricresol formalin, another formaldehyde preparation, contains 10% tricresol and 90% formaldehyde. Therefore all these preparations have a formaldehyde content well above the 10% normally used for fixation of pathologic specimens. Formaldehyde is volatile and releases antimicrobial vapors if applied on a cotton pellet for pulp chamber disinfection. All these formaldehyde preparations are potent toxins with an antimicrobial effectiveness much lower than their toxicity. The formaldehyde in contact with tissue in the pulp and periapical tissues is transported to all parts of the body. Considering the outright toxic and tissue destructive effects and the mutagenic and carcinogenic potential, no clinical reason exists to use formocresol as an antimicrobial agent for endodontic treatment. The alternatives are better antiseptics with significantly lower toxicity. Clinicians who might still contemplate using formaldehyde in practice are referred to Chapter 11 for a discussion of the legal implications of this unwise decision. Halogens Chlorine has been used for many years to irrigate the root canals. It also is sometimes used as an intracanal dressing in the form of Chloramine-T. Iodine, in the form of iodine potassium iodide (IKI), is a very effective antiseptic solution with a low tissue toxicity. One in vitro study showed that IKI (i.e., IKI 2%) penetrated deeper than 1000 μm of dentin in 5 minutes. IKI is an effective disinfectant for infected dentin and can kill bacteria in infected dentin in 5 minutes in vitro. Iodine potassium iodide releases vapors with a strong antimicrobial effect. The solution can be prepared by mixing 2 g of iodine in 4 g of potassium iodide; this mixture then is dissolved in 94 ml of distilled water. Tincture of iodine (5%) has proved to be one of the few reliable agents for disinfection of rubber dam and tooth surfaces during the preparation of an aseptic endodontic workfield. Calcium Hydroxide Hermann introduced the use of calcium hydroxide in endodontics in 1920. Although its use was well documented for its time, the clinical applications over the next 25 years were not well known. Calcium hydroxide cannot be categorized as a conventional antiseptic, but it kills bacteria in the root canal space. It has been routinely used by many clinicians over the past 40 years. The value of calcium hydroxide in endodontic treatment of necrotic, infected teeth is now well documented. Calcium hydroxide normally is used as a slurry of calcium hydroxide in a water base. At body temperature, less than 0.2% of the calcium hydroxide is dissolved into Caand OH- ions. Because Ca(OH)2 needs water to dissolve, water should be used as the vehicle for the calcium hydroxide paste. In contact with air, calcium hydroxide forms calcium carbonate (CaCO3). However, this is an extremely slow process of little clinical significance. Calcium hydroxide paste with a significant amount of calcium carbonate feels granular because the carbonate has a very low solubility. Some have suggested using Cresatin or camphorated parachlorophenol as the mixing vehicle. Mixing with Cresatin results in the formation of calcium cresylate and acetic acid, whereas mixing with camphorated parachlorophenol results in calcium parachlorophenolate. In both cases hydrolysis is inhibited, and the advantageous high pH is not reached. Calcium hydroxide is a slowly working antiseptic. Direct contact experiments in vitro show that a 24-hour contact period is required for complete killing of enterococci. In clinical experimentation, 1 week of intracanal dressing has been shown to safely disinfect a root canal system. A study of 42 patients found that sodium hypochlorite irrigation reduced the bacteria level by only 61.9%, but use of calcium hydroxide in the canals for 1 week resulted in a 92.5% reduction. These researchers concluded that Ca(OH)2 should be used in infected cases to more predictably obtain healing. In addition to killing bacteria, calcium hydroxide has the extraordinary ability to hydrolyze the lipid moiety of bacterial lipopolysaccharides (LPS), thereby inactivating the biologic activity of the lipopolysaccharide and reducing its effect. This is a very desirable effect because dead cell wall material remains after the bacteria have been killed and can continue to stimulate inflammatory responses in the periradicular tissue. Calcium hydroxide may be mixed with sterile water or saline; this formula is also available commercially from a number of manufacturers in sterile, single-dose packages (e.g., Calasept [J.S. Dental, Ridgefield, CT]; SteriCal [Centrix, Shelton, CT]; and DT Temporary Dressing, [Global Dental Products, North Bellmore, NY]) .The mixture should be thick to carry as many calcium hydroxide particles as possible. This slurry is best applied with a lentulo spiral. For maximal effectiveness the root canal must be filled homogeneously to the working length. Saturated calcium hydroxide solution mixed with a detergent is an effective antimicrobial agent suitable for irrigation. Bioactive Glass Research is underway in the use of bioactive glass as an intracanal medicament. In one study the glass used was composed of 53% SiO2 (w/w), 23% Na2O, 20% CaO, and 4% P2O5 and was prepared from reagent grade Na2CO3, CaHPO4, 2H2O, CaCO3, and Belgian sand. When used in root canals, bioactive glass was found to kill bacteria, but the mechanism of action was not pH related and dentin did not seem to alter its effect. Some new obturating materials (e.g., Resilon [Pentron Clinical Technologies, Wallingford, CT]) contain bioactive glass. Superoxidized Water Superoxidized water is saline that has been electrolyzed to form superoxidized water (hypochlorous acid and free chlorine radicals, supplied as Sterilox [Sterilox Technologies, Radnor, PA]). This solution is nontoxic to biologic tissues yet able to kill microorganisms. A study investigating the cleaning of endoscopes found that the solution killed microbes at a level equivalent to glutaraldehyde and superior to ozonated water and 0.05% chlorhexidine. However, the solution’s effectiveness was reduced by contact with albumin. These researchers concluded that superoxidized water was effective only after the endoscopes had been mechanically cleaned. In addition to its surface disinfection capability, superoxidized water has been shown to have potential as an endodontic irrigating solution. However, additional work is needed to confirm this result.