Meeting CMS Requirements for Coverage of Infection Control at
advertisement

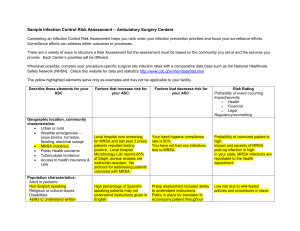
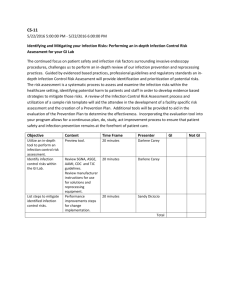
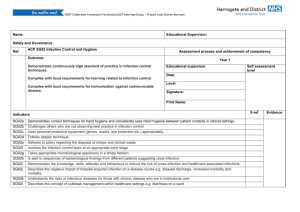
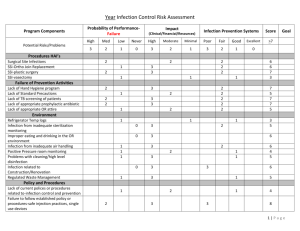
Meeting CMS Requirements for Coverage of Infection Control at Ambulatory Endoscopy Centers Laura Strohmeyer RN, CGRN, CASC Clinical Director, GI, AmSurg Objectives Review CMS Conditions for Coverage (CfC) on Infection Control as they pertain to Endoscopy Ambulatory Centers Examine common CMS deficiencies occurring at Endoscopy Ambulatory Centers Identify strategies to meet Infection Control CfC’s at Endoscopy Ambulatory Centers Endoscopy in the News January 2008- Hepatitis C outbreak in Nevada caused by reusing syringes, vials May 2009- Five patients have tested positive for HIV and 33 have tested positive for hepatitis since February, when the VA started notifying more than 11,000 people treated at three VA medical centers to get follow-up blood checks because they could have been exposed to infectious body fluids. The hospitals are in Miami, Murfreesboro, Tenn., and Augusta, Ga. Other Go to www.MyEndoSite.com for more Endoscopy in the News, Guidelines, and other reprocessing information Nevada ASC Problems CMS Conditions for Coverage on Infection Control 416.51 (Q-240): The ASC must maintain an infection control program that seeks to minimize infections and communicable diseases. 416.51a (Q-241): The ASC must provide a functional and sanitary environment for the provision of surgical services by adhering to professionally acceptable standards of practice. 416.51b (Q-242): The ASC must maintain an ongoing program designed to prevent, control, and investigate infections and communicable diseases. In addition, the infection control and prevention program must include documentation that the ASC has considered, selected, and implemented nationally recognized infection control guidelines. 416.51b1 (Q-243): The program is under the direction of a designated and qualified professional who has training in infection control. 416.51b2 (Q-244): The program is an integral part of the ASC’s quality assessment and performance improvement program. 1 416.51b3 (Q-245): The program is an responsible for providing a plan of action for preventing, identifying and managing infections and communicable diseases for immediately implementing corrective and preventative measures that result in improvement. Common CMS deficiencies Infection Control Program Don’t have a written plan Policy, goals Board meeting minutes QAPI minutes Focus studies Infection Control Professional o Don’t have one person identified o No training or not enough o No ongoing training o No job description o No documentation Infection Control Guidelines o None identified o Not approved by Board o Not followed Administration of Drugs o Syringes prepared ahead of time o Medications drawn up > 1 hour o Syringes not labeled o IV bags prepared ahead > 1 hour Sanitary Environment o Don’t know kill time for surface disinfectants o Not observing dry time o Clean vs. dirty o Hand Hygiene, PPE, Procedure room doors open during procedures Scope Reprocessing Deficiencies o Not following manufacturer’s instructions o No designated clean vs. dirty o Re-use of single use devices (bite blocks, polyp traps, dilation syringes) o No competencies, or not done in last 12 months o Scopes not contained during transport/ improper PPE o No designated clean and dirty areas o Reuse of single-use disposable brushes/sponges o Reusing enzymatic solution/rinse water; mixed incorrectly 2 o Not cleaning work surfaces between scopes o Incorrect scope storage- no scope cabinet, storing with valves or video cap o Unfamiliar with manufacturer instructions Enzymatic cleaner- dilution, soak time High-level disinfectant- time, temp , dates MEC test strips- checked with each use, dip time, read time, QC Scope washers- settings: cycle time, temp, PM’s Flushing pumps (Procedure and scope room)- tubings changed, decontamination, flow verification Water bottles- HLD or sterilized, sterile water only Scopes- follow most recent instructions Strategies to Meet CMS Infection Control CfC’s at Ambulatory Endoscopy Centers Infection Control Program Program Setup o Infection Control Program o Nationally Recognized Guidelines Training o Infection Control Professional o Staff training, credentialed staff Surveillance o Audit staff competency and compliance o Track patient/employee infections\ Guidelines Review CMS guidelines (interpretive guidelines) Search CMS Ambulatory Surgery Center Guidelines o CMS- Part 416- Appendix L- Guidance for Surveyors Ambulatory Surgery Centers https://www.cms.gov/GuidanceforLawsAndRegulations/02_ASCs.asp Appendix L of the State Operations Manual o Infection Control Surveyor Worksheet CDC- Guide for Infection Prevention in the Ambulatory Setting: Minimum Expectations for Safe Care Program Setup Evaluate current Endoscopy Infection Control Issues o Scope Reprocessing o Spaulding criteria High level disinfection Sterilization o Safe Injection Practices o Rapid turnover Identify Infection Control Professional o Job description o Board Approval 3 Surveillance of patient and employee infections Annual goals and evaluation of plan Nationally Recognized Guidelines: Choose guidelines that pertain to your center. Society of Gastroenterology Nurses and Associates (SGNA) www.sgna.org o SGNA Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes 2009 o SGNA Position Statement on Reprocessing of Water Bottles Used During Endoscopy 2009 o SGNA Guideline for Use of High Level Disinfectants & Sterilants for Reprocessing Flexible Gastrointestinal Endoscopes 2007 American Society for GI Endoscopy o Infection Control during GI Endoscopy o Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011 o ASGE- Reprocessing failure APIC: Position Paper: Safe Injection Practices CDC: Hand Hygiene, Personal Protective Equipment, Environmental cleaning Training Infection Control Professional o APIC Membership o SGNA Membership o Conferences APIC: Infection Prevention for ASC’s: Meeting CMS Conditions for Coverage o Webinars o AORN: ASC Infection Prevention Course (online) o Certification (state specific) o Ongoing: Stay informed of updates Staff o Review of infection control policies o Review of guidelines o Bulletin Boards o Posters o Staff meetings Physicians, anesthesia, contracted staff Documentation Surveillance- Patients Track suspected and reported infections, ask on post-op calls, may take months to identify o Phlebitis o Diarrhea o Fever Infection Control Breech Scope reprocessing 4 Sterilization Infection Control Outbreaks Surveillance- Personnel Track reported infections o GI infections o Flu o MRSA Prevention o Hepatitis B Immunizations o TB skin tests o Flu vaccine Compliance Competencies o Scope Reprocessing o Sterilization Audits o Scope Reprocessing o Sterilization o Hand Hygiene o Safe Injection Practices Reporting Staff Meetings Quality Assurance Performance Improvement o Infection Control Report o Infection Control Plan and evaluation o Infection Control focus studies o Policies and Procedures o Infection Control outbreak, concerns Governing Board 5